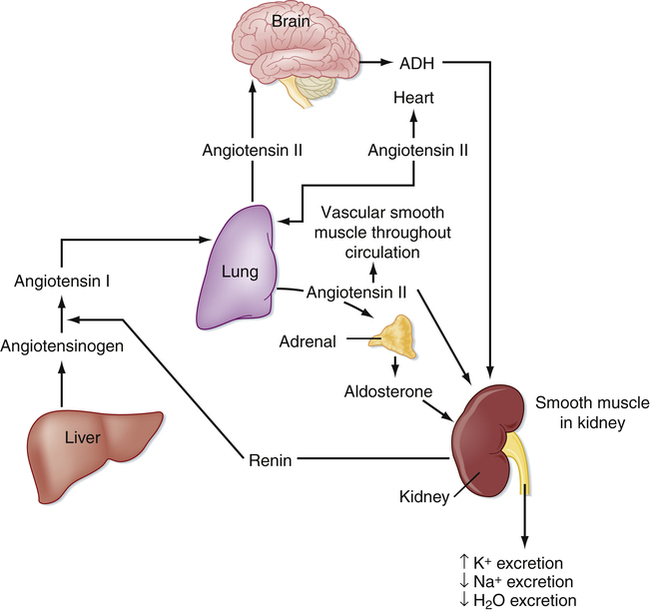
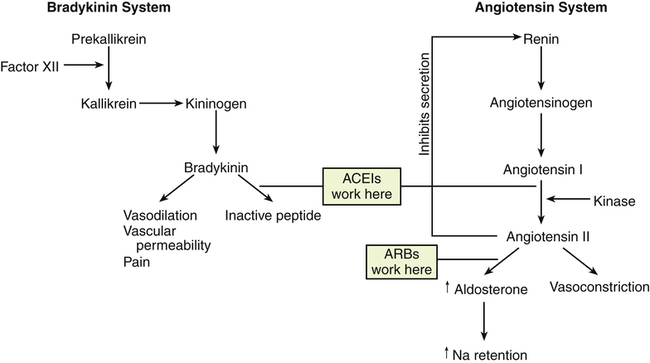
Chapter 22 Lisinopril is the key drug because it is the most commonly used. Two types of angiotensin receptors have been identified: AT1 and AT2. Most of the biologic effects of angiotensin II are mediated by the AT1 receptor. AT2 receptors may exert antiproliferative and vasodilatory effects. ACEIs block angiotensin-converting enzyme (ACE), which is responsible for the conversion of angiotensin I to angiotensin II. Angiotensin II is a potent vasoconstrictor and is a stimulus for aldosterone release from the adrenal glands (Figure 22-1). Reduction in aldosterone secretion results in less water absorption and sodium/potassium exchange in the distal renal tubule, causing a slight increase in serum potassium. ARBs block the effects of angiotensin II by blocking the binding of angiotensin II to its receptors. They do not affect bradykinin (Figure 22-2). Receptor affinity is highest by candesartan > irbesartan > eprosartan > telmisartan> valsartan > losartan. ARBs differ from ACEIs in the following four respects: 1. ARBs are more active against AT1 receptors than are ACEIs. 2. ACE inhibition is not associated with increased levels of angiotensin II, as are ARBs. 3. ACEIs may increase angiotensin I levels. 4. ACEIs increase levels of bradykinin (which may contribute to their side effects) in contrast to ARBs. Whether these differences translate to significant clinical outcomes is unknown. For information on standardized treatment guidelines, for evidence supporting these guidelines, and for information on nonpharmacologic treatment for patients with hypertension, MI, chronic heart failure, and diabetic nephropathy, see the related chapters: Chapters 17, 18, 19, and 53. In this chapter, only pharmacologic treatment with ACEIs and ARBs is discussed. ACEIs have similar therapeutic and adverse reactions. They differ basically in terms of pharmacokinetics (Table 22-2). Some are provided as prodrugs that must be metabolized by the liver to the active drug. The duration of hypotensive effects is critical. Many products claim that they provide 24-hour protection, but their effects may wear off within 24 hours. Blood pressure (BP) should be checked shortly before the time of administration to ensure 24-hour BP control. These agents differ in terms of tissue distribution, and this may result in differences in the renin-angiotensin systems affected. Except for fosinopril, these agents are cleared predominantly by the kidney. ACEIs generally are considered safe and effective in patients with mild to moderate renal impairment; however, dosage reduction is required in patients whose renal clearance is diminished. Fosinopril, lisinopril, and ramipril are eliminated by both hepatic and renal mechanisms, and they have the ability to compensate for renal dysfunction by shifting to hepatic elimination. Dehydration and renal insufficiency increase the risk of elevated K when an ACEI is started. TABLE 22-2 Pharmacokinetics of ACEI Agents
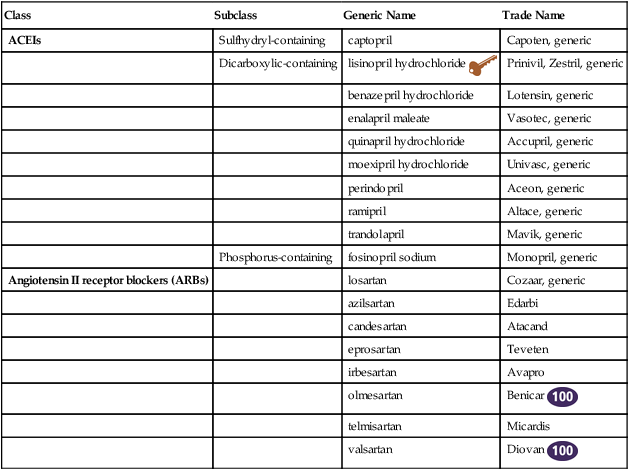
ACE Inhibitors and Angiotensin Receptor Blockers
Class
Subclass
Generic Name
Trade Name
ACEIs
Sulfhydryl-containing
captopril
Capoten, generic
Dicarboxylic-containing
lisinopril hydrochloride ![]()
Prinivil, Zestril, generic
benazepril hydrochloride
Lotensin, generic
enalapril maleate
Vasotec, generic
quinapril hydrochloride
Accupril, generic
moexipril hydrochloride
Univasc, generic
perindopril
Aceon, generic
ramipril
Altace, generic
trandolapril
Mavik, generic
Phosphorus-containing
fosinopril sodium
Monopril, generic
Angiotensin II receptor blockers (ARBs)
losartan
Cozaar, generic
azilsartan
Edarbi
candesartan
Atacand
eprosartan
Teveten
irbesartan
Avapro
olmesartan
Benicar ![]()
telmisartan
Micardis
valsartan
Diovan ![]()
Mechanism of Action
Treatment Principles
Drug (Active Metabolite)
Effect of Food on Absorption
Onset of Action
Duration of Action
Time to Peak Concentration
Half-Life
Protein Bound
Metabolism
Excreted Unchanged
captopril (Capoten)
Reduced absorption (30%-40%)
0.25 hr
6 hr
1-1.5 hr
2 hr; 20-40 hr (anuria)
25%-30%
50%
Urine, 40%-50%
lisinopril (Zestril)
None
1 hr
24 hr
6 hr
12 hr
25%
—
Urine, 100%
benazepril (Lotensin)
None
1 hr
24 hr
2 hr
10-11 hr
>95%
Liver; metabolized to active drug
Nonrenal (biliary, 12%) and renal (8%)
enalapril (Vasotec)
None
1 hr
12-24 hr
4-6 hr
11 hr
—
Liver; metabolized to active drug
Urine, 60%-80%; some feces
quinapril (Accupril)
Reduced
1 hr
24 hr
2-4 hr
25 hr
97%
Liver; metabolized to active drug
Renal, 96%
moexipril (Univasc)
Reduced
1.5 hr
24 hr
3-6 hr
2-10 hr
50%
Liver; metabolized to active drug
Urine, 52%
ramipril (Altace)
Reduced
1-2 hr
24 hr
1.1-4.5 hr
13-17 hr
56%
Liver; metabolized to active drug
Urine (60%) and feces (40%)
trandolapril (Mavik)
Reduced
4 hr
24 hr
—
5 hr
80%
Liver, 14%
Urine, 33%; feces, 56%
fosinopril (Fosinoprilat)
None
1 hr
24 hr
3 hr
12 hr
95%
Liver; metabolized to active drug
Urine (50%) and feces (50%)
losartan (Cozaar)
Well absorbed
1 hr
12-24 hr
3-4 hr
6-9 hr
99%
CYP450 2C9 substrate and 3A4 substrate active metabolites
Urine, 4%-6%
candesartan
Well absorbed
2-3 hr
>24 hr
6-8 hr
9 hr
99%
Liver
Urine (26%) and feces (67%)
eprosartan
Reduced absorption
1 hr
12-24 hr
1-2 hr
5-9 hr
98%
Liver
Urine (7%) and feces (90%)
irbesartan (Avapro)
Rapidly absorbed
1-2 hr
>24 hr
1.5-2 hr
11-15 hr
90%
Liver
Urine (20%) and feces (80%)
telmisartan
Reduced
1-2 hr
24 hr
0.5-1 hr
24 hr
99.5%
Liver
Feces, 97%
valsartan (Diovan)
Reduced
2 wk
24 hr
2-4 hr
6 hr
95%
Liver, 20%; not CYP450
Urine (13%) and feces (83%) ![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


ACE Inhibitors and Angiotensin Receptor Blockers
Only gold members can continue reading. Log In or Register to continue