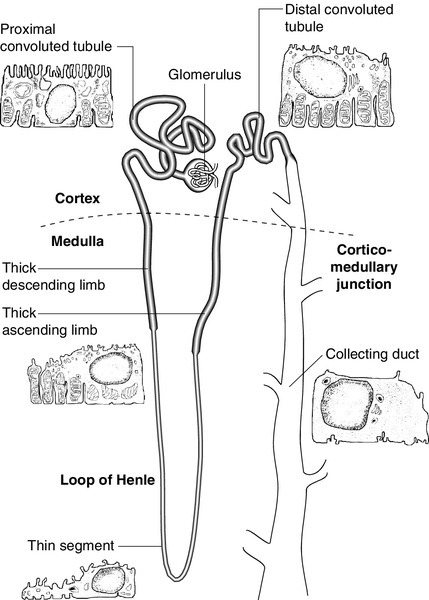
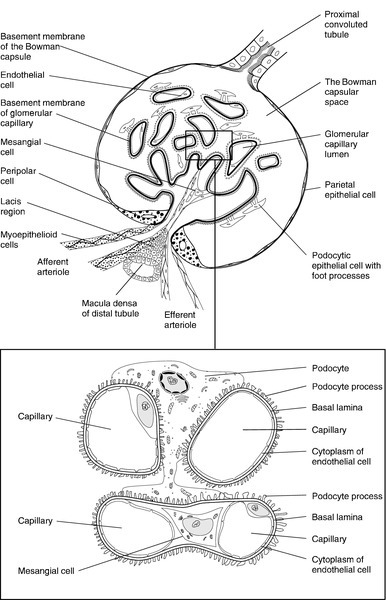
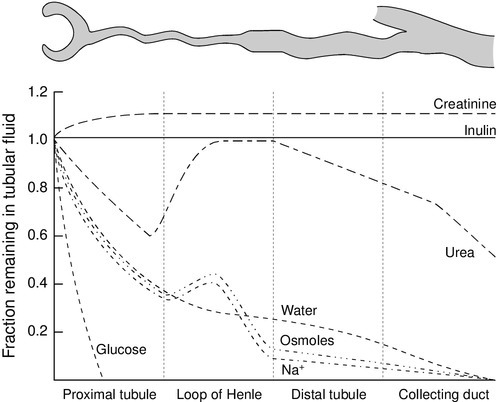
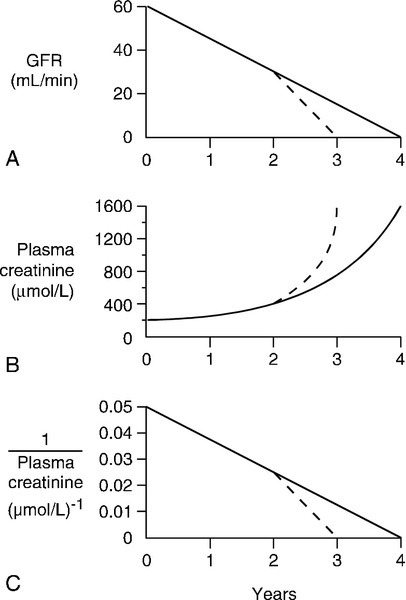
CHAPTER 7 David Makanjuola; Marta Lapsley CHAPTER OUTLINE Renal blood flow and its control RENAL DISEASE AND ITS PRESENTATION Manifestations of renal disease Diseases affecting the kidneys THE ASSESSMENT OF RENAL FUNCTION Biochemical tests of renal function ACUTE KIDNEY INJURY (ACUTE RENAL FAILURE) Obstructive (postrenal) kidney injury Acute kidney injury in the setting of chronic kidney disease Metabolic consequences and management of acute kidney injury Aetiology and pathogenesis of chronic kidney disease Endocrine control of salt and water balance Carbohydrate metabolism and lipid metabolism The kidneys are located against the posterior abdominal wall on either side of the vertebral column. They lie anterior to the diaphragm and various muscles; their anterior surfaces are covered by parietal peritoneum. The left kidney is posterior to the stomach, pancreas, spleen and descending colon, and the right to the liver, the second part of the duodenum and the ascending colon. Their superior poles are covered by the suprarenal (adrenal) glands. In the adult, the kidneys are about 11–13 cm long, 6 cm wide and 4 cm thick. They weigh approximately 150 g each, yet together receive about 25% of the resting cardiac output. This blood is supplied by the renal arteries, which are branches of the aorta. Venous drainage is into the renal veins, which drain into the inferior vena cava. The kidneys are innervated by sympathetic nerves arising from the sympathetic chains and by parasympathetic fibres arising from the vagus nerve. Each kidney is covered by a poorly distensible capsule. This limits the swelling that can occur during acute inflammation, and in such circumstances there is an increase in tissue pressure that tends to decrease the glomerular filtration rate (GFR). Congenital absence of one kidney occurs in approximately 1 in 2400 individuals; this is only of significance if renal surgery is contemplated for any reason. The remaining kidney usually undergoes compensatory hypertrophy. Another common (approximately 1 in 10 000) congenital anomaly is the single ‘horseshoe’ kidney, in which the lower poles of the potential two kidneys are conjoined; renal function is normal but, occasionally, ureteric obstruction may predispose to calculus formation and infection. Each kidney contains approximately one million functional units, called nephrons. These are tubular structures, consisting of various histologically and functionally distinct elements. Each nephron has a single glomerulus, which is located in the outer part of the kidney (the cortex) and is responsible for the ultrafiltration of the blood. The remainder of the nephron consists of several contiguous tubular structures that progressively modify the composition of the ultrafiltrate before it is passed out as urine. These structures (Fig. 7.1) are: • the proximal convoluted tubule, also located in the cortex • the loop of Henle, which has the configuration of a hairpin and extends into the deeper part of the kidney (the medulla), then doubles back on itself to return to the cortex • the distal convoluted tubule, located in the cortex • the collecting duct, which extends down through the medulla to the renal papillae, whence the urine drains into the renal pelvis. The renal pelvices are drained by the ureters, and the right and left ureters themselves drain into the urinary bladder, where urine is stored prior to voiding. Each glomerulus consists of a tuft of capillaries that protrudes into the dilated, blind end of the nephron (the Bowman capsule) (Fig. 7.2). The nephron is composed of epithelial cells, and these and the endothelial cells of the glomerular capillaries are separated by a basement membrane. This visceral basement membrane is continuous with a parietal basement membrane associated with the epithelial cells forming the outer part of the Bowman capsule, which are continuous with the epithelium of the proximal convoluted tubule. The microstructure of the glomerulus is described in detail in Chapter 8. The glomerular filtrate is formed by the passage of fluid from the capillaries through fenestrations in the endothelial cells and through the basement membrane. This is covered on its epithelial aspect by the interdigitating foot processes of the epithelial cells, or podocytes, and the ultrafiltrate passes across a thin membrane (the epithelial slit diaphragm) into the filtration slits between the foot processes and then into the Bowman space, which is continuous with the lumen of the nephron. The selective retention of different constituents of the blood occurs at different stages. The endothelial fenestrations are too small to permit significant passage of erythrocytes, leukocytes or platelets; the major barrier to the filtration of proteins is mechanical and provided by the inner layer of the basement membrane, but the anionic surface layer also selectively retards anionic proteins. Finally, the foot processes and slit diaphragms also act as a mechanical barrier. The relative rate of progression of the constituents of the plasma into the Bowman space, therefore, depends on molecular mass, shape and charge. Glomeruli also contain mesangial cells. These stellate cells are contractile, are involved in the regulation of glomerular filtration and have phagocytic properties. The basement membrane does not extend between the mesangial cells and endothelial cells. This facilitates their ability to phagocytose large particles from the plasma. Their ability to ingest immune complexes plays an important part in the pathogenesis of certain forms of glomerular disease. This structure is about 15 mm long and is composed of a single layer of inter-digitating epithelial cells united at their apices by tight junctions. The luminal surfaces of these cells have microvillous brush borders that provide the large surface area required for the absorptive function of the proximal tubule. The proximal tubule drains into a short straight segment directed towards the outer medulla and continuous with the descending limb of the loop of Henle. The descending limb of the loop of Henle (Fig. 7.1) is composed of flat epithelial cells. The majority of the loops, whose glomeruli lie in the outer part of the cortex, are relatively short, but those with more deeply situated glomeruli have longer loops (up to 14 mm), which extend down into the medullary pyramids. The thin descending limb turns back on itself, ascending towards the medulla, and the epithelial cells become cuboid, rich in mitochondria and have an invaginated luminal surface. The thick ascending limb extends towards the glomerulus of the same nephron and then leads into the distal convoluted tubule. The distal convoluted tubule is about 5 mm long and is made up of typical absorptive epithelial cells, although without a brush border. It passes close to the afferent and efferent arterioles of the same nephron. The tubular epithelial cells in this area have a special function in relation to the control of renin secretion and are known as the macula densa. Renin is secreted by myoepithelioid juxtaglomerular cells in the walls of the afferent arterioles. These cells, together with those of the macula densa and some other nearby cells (lacis cells), constitute the juxtaglomerular apparatus. The distal convoluted tubule also contains intercalated (I) cells, responsible for the secretion of acid. The collecting ducts are composed of I cells and also principal (P) cells, which become permeable to water under the influence of vasopressin. The ducts descend from the cortex down through the medulla, opening to the renal pelvis from the renal papillae. In addition to the cells of the nephron and blood vessels, the kidneys contain medullary interstitial cells. These characteristically contain lipid droplets and secrete prostaglandins. Blood leaving glomerular capillary tufts passes not into venules, but into efferent arterioles. Those draining the cortical glomeruli divide into a capillary plexus that enmeshes the renal tubules. Those draining the juxtamedullary glomeruli form the vasa recta; these are bundles of vessels that plunge into the medulla to varying depths, forming capillary plexi that envelop the loops of Henle and then rejoin to form the ascending vasa recta, which eventually drain into the renal veins. The vasa recta form a countercurrent exchange system (see below), which is essential to the formation of both concentrated and dilute (with respect to plasma) urine. The principal functions of the kidneys are to excrete water-soluble (mostly nitrogenous) waste products and toxins from the body, and to control the volume and composition of the extracellular fluid (ECF). The kidneys also have important endocrine functions. It is perhaps surprising that the kidneys produce some 170 L of glomerular filtrate per 24 h, but only 1–2 L of urine. The bulk of the solvent and solute filtered is reabsorbed, but the control of the processes responsible gives considerable scope for the composition and volume of the urine to be adjusted to meet the physiological needs of the individual. The physiology of the kidneys is well described in many standard physiology textbooks and will not be recounted in detail in this chapter. Rather, a brief summary is given and certain features that are pertinent to an understanding of the investigation of renal function and the mechanisms and consequences of renal impairment are emphasized. At rest, 20–25% of the cardiac output flows through the kidneys (1.1–1.3 L/min). The bulk of this blood perfuses the cortex; indeed, the flow rate to the medulla is actually less than to most other tissues. The process of glomerular filtration is essential to renal function and is dependent on an adequate renal perfusion pressure. Autoregulatory mechanisms allow maintenance of renal blood flow and GFR within narrow limits in the face of a wide variety of external variables, including arterial pressure, venous pressure, ureteric hydrostatic pressure and plasma oncotic pressure. Renal blood flow and GFR are independent of mean arterial blood pressure over the range 80–200 mmHg. This is achieved by intrinsic, and humorally and neurally mediated, alterations in the tone of the renal blood vessels and, in particular, of the efferent glomerular arterioles. Renal hypoperfusion stimulates the release of renin from juxtaglomerular cells. This enzyme converts circulating angiotensinogen to angiotensin I, which is in turn converted by angiotensin-converting-enzyme to angiotensin II, a powerful vasoconstrictor. This contributes to the maintenance of systemic blood pressure and, in the kidney, by causing efferent arteriolar vasoconstriction, helps to maintain the intraglomerular pressure despite a reduction in perfusion pressure. Various other mechanisms are involved in the maintenance of systemic blood pressure, but in the poorly perfused kidney, release of prostaglandins causes vasodilatation which, at least in mild hypotension, helps to maintain renal blood flow. A perfusion pressure of at least 50–60 mmHg is required to overcome the combined hydrostatic and oncotic pressures that oppose filtration; if mean arterial pressure falls below 80 mmHg, renal blood flow and GFR decline rapidly. Glomerular filtration is fundamental to the production of urine and to the homoeostatic functions of the kidney. Techniques for measuring the GFR, the amount of filtrate formed per unit time, are described in a later section (p. 131). The glomerular filtration rate is determined by the balance of pressures across the filtration barrier in the glomerulus, and the physical nature and extent of the barrier itself. The forces include the difference between afferent and efferent glomerular arteriolar pressures (promoting filtration) opposed by the difference in osmotic pressures between the ultrafiltrate and the plasma, and the hydrostatic pressure in the Bowman space. The net filtration pressure is normally approximately 15 mmHg at the afferent end of the capillaries, falling to zero at the efferent ends. Factors influencing the GFR are summarized in Box 7.1. A decrease in the area available for filtration can occur in many renal diseases in which there is glomerular damage. It can also result from contraction of mesangial cells mediated by agents such as angiotensin II, vasopressin, noradrenaline (norepinephrine), thromboxane A2 and prostaglandin F2; dopamine, natriuretic peptides and prostaglandin E2 have opposite effects. A reduction in filtration area may be an important physiological mechanism for reducing GFR and thus conserving fluid. The proximal tubules are responsible for the active reabsorption of the bulk of filtered solute, accompanied by an iso-osmotic amount of water. Thus, virtually all the filtered glucose, amino acids, bicarbonate and potassium are absorbed here, together with some two-thirds of the filtered sodium. Absorption of solutes is an active, energy-requiring process and is isotonic, so that an equivalent amount of water is also absorbed and the fluid entering the descending limb of the loop of Henle is isotonic with plasma. Transport occurs by way of ion channels, exchangers, co-transporters and pumps; filtered proteins are taken up into tubular cells by endocytosis and broken down into their constituent amino acids. Transport mechanisms have finite capacities and these may be exceeded in some circumstances. For example, glucose begins to be excreted in the urine if the plasma concentration exceeds about 10 mmol/L. Isolated or generalized disorders of renal tubular function result in the excretion of solutes normally reabsorbed in the proximal tubule even at normal plasma concentrations, as discussed further in Chapter 9. The sodium content of the ECF is the primary determinant of ECF volume, the maintenance of which is critical to life. Given that about 26 000 mmol of sodium are filtered at the glomeruli each day (equivalent to some 8–9 times the total body sodium), it is clearly essential that most of the filtered sodium is reabsorbed. Indeed, the bulk of sodium reabsorption is obligatory. However, an increase in GFR (which potentially could lead to a massive increase in sodium excretion) results in increased proximal tubular sodium absorption and vice versa. This process, termed glomerulo-tubular balance, is thought to be mediated largely through changes in the oncotic pressure in the peri-tubular capillaries. This increases when the GFR increases, owing to the increase in the volume of water removed from the plasma. The re-absorption of several other solutes is affected similarly. Normal kidneys are capable of excreting between virtually zero and more than 400 mmol of sodium per 24 h. The fine control of sodium excretion is achieved in the distal convoluted tubule, where sodium reabsorption is stimulated by aldosterone. Most of the filtered bicarbonate is absorbed in the proximal convoluted tubule, although indirectly. Hydrogen ions are generated in the tubular cells and are secreted into the tubular lumen (mainly in exchange for sodium) where they combine with the filtered bicarbonate ions. Bicarbonate ions are formed pari passu with hydrogen ions and are co-transported with sodium across the basolateral membranes of the tubular cells into the interstitial space (see Chapter 5). Some substances are secreted into the urine from the proximal tubule. Examples include penicillins, creatinine, certain steroids and their glucuronides and derivatives of hippuric acid, for example p-aminohippuric acid (PAH). This property of PAH allows physiologists to use measurements of PAH clearance to determine renal plasma flow, but this is not an investigation that is used clinically. This structure provides the countercurrent multiplier that generates the medullary hypertonicity that is essential for the regulation of water excretion and the production of concentrated urine. Essentially, the process involves the active transport of solute (principally chloride and sodium ions) out of the thick ascending limb of the loop of Henle. This solute is not accompanied by water. As a result, the fluid within the lumen becomes hypotonic and the interstitial fluid surrounding the loop becomes hypertonic. Since water can diffuse out of the thin descending limb, but not the thick ascending limb, the net effect is that the fluid within the loop of Henle and the surrounding interstitial fluid become progressively hypertonic from the corticomedullary junction down into the medulla. There is further sodium and chloride absorption from the thick ascending limb. As water cannot diffuse out of the thick limb, the fluid entering the distal convoluted tubule is hypotonic with respect to plasma. The anatomical disposition of the vasa recta allows them to act as a countercurrent exchanger, maintaining (though not actively contributing to) the osmotic gradient. The flows of water and solutes in the nephron are summarized in Figure 7.3. Whereas 60–70% of the filtered loads of solute and water have been reabsorbed from the glomerular filtrate at the beginning of the loop of Henle, a further 15% of water is removed during passage through this structure, so that only 10–20% of filtered water and somewhat less solute reach the distal convoluted tubule. There is a reciprocal relationship between the rate of flow of fluid through the ascending limb of the loop of Henle and the first part of the distal convoluted tubule of individual nephrons, and the rate of glomerular filtration through the same nephron. Thus, a decrease in flow rate increases filtration and vice versa. This process tends to result in a constant load of solute being presented to the distal convoluted tubule. Feedback is mediated through constriction and dilatation of the afferent arteriole by factors such as renal prostaglandins, adenosine and the renin–angiotensin system, in response to changes in chloride absorption in the macula densa. This mechanism becomes more sensitive when ECF volume is decreased and vice versa. Tubuloglomerular feedback should not be confused with glomerulotubular balance, the change in proximal tubular sodium reabsorption in response to changes in GFR that has been discussed above and in Chapter 4. Urea has an essential role in the generation of medullary hypertonicity. The thick ascending limb of the loop of Henle is permeable to urea (and sodium) but not to water. Movement of urea down its concentration gradient into the interstitium contributes significantly to medullary hypertonicity; the inner medullary portion of the collecting duct (see below) is also permeable to urea. The poor concentrating ability of the kidneys in the newborn is due to the decreased availability of urea to maintain medullary hypertonicity. In adults, renal concentrating power is dependent on an adequate protein intake (the source of urea) and is lower on a low protein diet. This part of the nephron is responsible for the ‘fine tuning’ of urinary composition. Aldosterone stimulates sodium reabsorption, generating an electrochemical gradient that allows the secretion (and thus excretion) of potassium and hydrogen ions. Normal distal tubular function is essential to the maintenance of hydrogen ion homoeostasis. These structures extend from the ends of the distal convoluted tubules to the renal papillae. Their main function is to permit the reabsorption of solute-free water and thus regulate the osmolality of the urine. This is achieved through the action of vasopressin (antidiuretic hormone). The cells of the collecting ducts are normally impermeable to water. Under the influence of vasopressin, aquaporin-2 water channels are inserted into the tubular wall, such that they become permeable, allowing water to move out of the hypotonic fluid in the tubular lumen into the hypertonic interstitium, so producing concentrated urine. In the absence of vasopressin, no water is removed and the urine is hypotonic. The extremes of achievable urine osmolality are 30–1400 mmol/kg. When maximally dilute, as much as 13% of the filtered water is excreted and the rate of urine production is about 16 mL/min; when maximally concentrated, < 0.5% of filtered water is excreted and urine production is as little as 500 mL/24 h. Some solute is also absorbed in the collecting ducts. In the cortical portion, sodium reabsorption takes place in exchange for potassium and hydrogen ions, as in the distal convoluted tubules. The medullary segment is partially permeable to urea, which moves passively out into the interstitium and helps to maintain the high osmolality of the medulla. While the maximal urine flow rate when a water load is being excreted is approximately 16 mL/min (assuming an average solute load), higher rates are attainable if there are increased quantities of non-reabsorbed solutes in the tubules. This osmotic diuresis is due to the direct osmotic effect of the solutes and to a secondary effect on sodium reabsorption. The retention of water in the lumen of the proximal tubules increases the concentration gradient against which sodium must be reabsorbed; the same factor limits sodium reabsorption in the thick ascending part of the loop of Henle, thus interfering with the generation of medullary hypertonicity. This is the explanation for the diuresis characteristic of hyperglycaemia. There are many specific renal diseases; these may present with clinical features clearly referable to the kidneys (e.g. a decrease or increase in urine output), but frequently have systemic manifestations (e.g. the ‘uraemic syndrome’ (see p. 142) and hypertension). The kidneys can also be involved in multisystem disease, for example diabetes mellitus, the connective tissue disorders and amyloidosis. There are relatively few cardinal manifestations of renal disease and none is specific to any one underlying disorder. They include anuria, oliguria and polyuria; respectively a complete absence, decreased production and excessive production of urine. Polyuria may come to notice only by virtue of causing excessive production of urine at night (nocturia). These are usually symptoms of disordered renal function, although anuria and oliguria can be secondary to obstruction of the urinary tract and polyuria can be due to extrarenal disease that impairs the ability of the kidneys to concentrate the urine. Urinary frequency (the passing of urine more frequently than normal, but in normal total 24 h volumes) must be distinguished from polyuria; it is often related to irritation of the urinary tract, for example by infection, but can also be due to prostatic enlargement. Irritation of the urinary tract is frequently accompanied by dysuria – pain on micturition. Patients with renal disease may experience loin pain or have tenderness in the loins. Renal or ureteric colic, an intermittent pain of considerable severity, is characteristic of the passage of urinary calculi. Patients with renal disease may present with systemic abnormalities, including hypertension, pyrexia, oedema and the ‘uraemic syndrome’. This latter term is used to describe the protean manifestations of renal failure. The urine may appear abnormal in renal disease; the causes are discussed in a later section (p. 130). Finally, renal disease may be revealed for the first time by the finding of a biochemical abnormality, particularly an elevated plasma creatinine or urea concentration, or positive urine dip-stick tests for protein or blood. Although patients with renal disease frequently present with an obvious functional abnormality, for example increased plasma creatinine or proteinuria, these are not specific to any one renal disease. A full discussion of the nature of individual renal diseases is beyond the scope of this chapter. Many renal diseases specifically affect one part of the kidney. Glomerulonephritis, which is often of immunological origin, can manifest by decreases in the GFR or changes in glomerular permeability (e.g. causing proteinuria). Glomerulonephritis may be of primary renal origin, or occur secondarily to an extrarenal disorder (see Chapter 8). A considerable number of conditions, both inherited and acquired, limited to the kidneys and having extrarenal manifestations, can cause disorders of renal tubular function. They are discussed in detail in Chapter 9. The kidneys can be affected by infection, tumours and infiltrative and degenerative disorders. Generalized disorders, particularly shock, hypertension, connective tissue diseases and diabetes, are also frequent causes of renal dysfunction. The kidneys are also susceptible to the effects of toxins, including many widely used drugs (e.g. the aminoglycoside antibiotics and some cytotoxic drugs), as well as industrial and other toxins, and exposure to such agents is another frequent cause of renal dysfunction. The major part of this chapter is devoted to a discussion of generalized renal failure, both acute and chronic. These are potentially life-threatening conditions and the clinical biochemistry laboratory plays a major part in their diagnosis and management. Many techniques are of value in the investigation of the kidneys and renal function. The major groupings of techniques are laboratory tests, particularly biochemical and immunological, radiological imaging and the histological examination of renal tissue usually obtained by percutaneous biopsy. Imaging techniques: standard radiography, ultrasonography, computed tomography, magnetic resonance imaging, the use of radionuclides and duplex scanning, can provide valuable anatomical information. Such techniques can be used to diagnose, for example, structural abnormalities, tumours, hydronephrosis, polycystic disease and the scarring of pyelonephritis. Radionuclide techniques can also provide information concerning renal function, particularly divided function (the separate function of the two kidneys) and glomerular function. Duplex scanning is a good screening test to assess renal blood flow before proceeding to magnetic resonance angiography or renal angiography. Useful immunological markers include the detection in serum of antiglomerular basement membrane antibodies (positive in Goodpasture syndrome), antineutrophil cytoplasmic antibodies (in vasculitis), antinuclear antibodies and double-stranded DNA antibodies (in systemic lupus erythematosus), complement components (in various types of glomerulonephritis) and antistreptolysin O antibodies (in post-streptococcal glomerulonephritis). Immunological techniques are also widely used in the histological examination of renal tissue. However, given the pivotal role of the kidneys in body fluid homoeostasis, it is not surprising that biochemical investigations that indicate derangements of homoeostasis are the most widely used tests of renal function. The remainder of this section covers such tests. The assessment of renal function should start with examination of the urine. Simple observations on the urine still contribute to the investigation of patients with suspected renal disease. To be valid, they must be made on a freshly passed sample. The normal colour of urine is due to urochrome pigments, and the deepness of the colouration, which can vary from a pale straw colour to deep amber, depends on urinary concentration. There are many causes of abnormal colouration of the urine, some of the more frequently encountered of which are indicated in Box 7.2. In some cases, the colour is pH dependent. The distinction between haemoglobin or myoglobin and other causes of a reddish-brown urine can simply be accomplished by testing the urine with a suitable reagent stick; both myoglobin and haemoglobin will give a positive reaction for ‘blood’. Turbidity in a fresh sample suggests infection, but can also be due to the presence of fat in patients with the nephrotic syndrome. The presence of chyle in the urine may cause turbidity so severe that the urine appears milky. Anything more than a scant amount of foaming when the urine is shaken, suggests proteinuria. The most frequent abnormal odour is that of ammonia, owing to the presence of urea-splitting microbes, either because the patient has a urinary infection or, in stale samples, because of contamination. Both of these give an indication of the concentration of the urine. When this needs to be known accurately, for example in tests of renal concentrating ability, measurements of osmolality are essential. A reagent stick assessment of specific gravity may sometimes help in the interpretation of a screening test for proteinuria, since false negative results can occur with very dilute urine and vice versa. However, these reagent sticks detect ionic species only, and underestimate the specific gravity if other substances, for example glucose, are present. Normal urine is acidic, except after meals. Measurements of urine pH are important in the investigation of renal stone formers and as part of a test of urinary acidification in the investigation of suspected renal tubular acidosis. Glycosuria can occur either because of an increase in blood glucose concentration or because of a low renal threshold for glucose, owing to impaired proximal tubular reabsorption. In the latter instance, the blood glucose concentration will be normal. Renal glycosuria is discussed further in Chapter 9. Proteinuria is an important feature of renal disease. Normal urine protein excretion is < 200 mg/24 h and is not detectable with standard reagent sticks (e.g. ‘Albustix’). Pathological proteinuria may be: • overflow (due to raised plasma concentrations of low molecular weight proteins) • glomerular (due to increased glomerular permeability to protein) • tubular (due to decreased tubular reabsorption of filtered protein or saturated reabsorption) • secreted (derived from the epithelium of the urinary tract). Further details of the causes, detection and investigation of proteinuria are discussed in Chapter 8 and will not be considered further here. Microscopic examination of the sediment obtained by centrifuging a freshly passed sample of urine frequently reveals a few cells (e.g. erythrocytes, polymorphonuclear leukocytes and cells derived from the kidney and urinary tract), hyaline (or rarely granular) casts composed of uromodulin (Tamm–Horsfall protein), and sometimes fat droplets, mucus threads and pigment granules. Increases in any of these may occur in renal diseases. Abnormal casts are also frequently present. These are accretions of uromodulin with other material, for example cells or cellular debris. Red cell casts imply haematuria from glomerular disease, and white cell casts, the presence of white cells within the renal tubules. In acute pyelonephritis, there is an increase in polymorphs and various types of cast, in contrast to lower urinary tract infection where polymorphs, but not casts, are increased. Microbiological examination in acute pyelonephritis will usually reveal bacteriuria. In acute glomerulonephritis, haematuria causes the urine to appear reddish-brown or smokey, and the sediment contains large numbers of red and white blood cells, and red cell, haemoglobin and other casts. In chronic glomerulonephritis, the amount of sediment is much less, but there is usually an excess of dysmorphic red blood cells, which suggests that red cells have passed through the glomerular membrane. Red cell and haemoglobin casts are sometimes present. Granular casts are seen in the urinary sediment in many renal diseases, but particularly in acute tubular necrosis; in this condition, casts containing tubular cells are also often present, but red cell casts are uncommon. Characteristic crystals may be seen when specific substances are present in excess or the pH of the urine promotes crystallization, for example, hexagonal cystine crystals, birefringent calcium oxalate crystals, calcium phosphate or uric acid crystals (see Chapter 9). Many other substances can be detected in the urine in appropriate circumstances, for example drugs or bilirubin. Their presence is due to the normal excretory function of the kidneys and is not a reflection of renal dysfunction, so they will not be considered further here. Glomerular filtration is essential to renal function, and investigations designed to measure the GFR are the most frequently performed tests of renal function. The factors that determine the GFR have been discussed above. Its measurement is based on the concept of clearance – the determination of the volume of plasma from which a substance is completely removed (the plasma is ‘cleared’ of the substance) by glomerular filtration during its passage through the kidney. Clearance is a theoretical concept – no substance is cleared completely from the plasma in this way – but can nevertheless be used as a valid measure of the GFR. For the clearance of a substance to equal the GFR, it must be freely filtered by the glomeruli and eliminated from the body solely by this route (e.g. it must not be metabolized by the liver or secreted into or reabsorbed from the urine). The substance should be non-toxic and accurate measurement should be easily available in a routine laboratory. The clearance of a substance requires measurements of the plasma concentration and urinary excretion rate: where U is the concentration of the substance in the urine, The critical properties of some of the substances used in clearance measurements to assess GFR are shown in Table 7.1. TABLE 7.1 Properties of substances used to assess glomerular filtration rate a The ideal substance would have all these properties. DTPA, diethylenetriaminepentaacetic acid. Clearance can also be used to determine renal plasma flow, using a substance such as p-aminohippuric acid, which is almost completely cleared from the blood in a single passage through the kidneys by a combination of glomerular filtration and secretion. Inulin is a plant polysaccharide that satisfies all the physiological criteria mentioned in the previous section. The measurement of inulin clearance remains the ‘gold standard’ for the estimation of GFR. However, it is a relatively complex procedure and, although test kits are commercially available, it is infrequently used in clinical practice. In essence, the test involves the injection of a bolus dose of inulin, followed by a maintenance infusion designed to produce a constant plasma concentration. Once this has been achieved, a series of timed urine samples is collected and blood is drawn for the measurement of inulin at the midpoints of the collection periods. The GFR is taken as the mean of the inulin clearances for each period. Creatinine is an endogenous substance, a normal product of muscle metabolism. Its rate of production is fairly constant from day-to-day, being determined by muscle bulk rather than by activity. Creatinine is removed from the body mainly by glomerular filtration and its clearance can be measured as an index of GFR, although in the UK, it is much less frequently used than previously. Creatinine is actively secreted into the urine, so that its clearance tends to overestimate the GFR. This effect is of little significance at normal filtration rates, the ratio (creatinine clearance/inulin clearance) being between 1.1 and 1.2, but in advanced renal disease the contribution of active secretion to the total amount of creatinine excreted in the urine becomes high in relation to the amount filtered, and creatinine clearance may significantly overestimate the true GFR. This is despite the fact that in established renal failure, bacterial degradation of creatinine secreted into the gut may contribute approximately 2 mL/min to the total clearance. Inaccuracies arising from methodological problems with creatinine measurement have largely been overcome, but the major cause of inaccuracy in the determination of GFR by creatinine clearance is the accurate measurement of urine volume. Traditionally, patients have been required to collect urine over a 24 h period. This is a convenient time, but requires the patient to void their urine completely at the beginning of the 24 h period and collect all the urine passed over the ensuing 24 h, making a final collection at the end of this period. The possibilities for mistakes resulting in an incomplete collection are considerable and even under ideal conditions, for example in a metabolic unit with highly motivated patients, the coefficient of variation for repeated measurements in the same individual is > 10%. The critical difference (the amount by which two estimates must differ to give a 95% probability that there has been a true change in GFR) is therefore > 33%. The accuracy of estimates of the GFR by measurement of creatinine clearance may be improved by making two or more consecutive 24 h urine collections, but this is often not practical even if it is acceptable to the patient. There is, however, nothing special about the 24 h period. The use of shorter periods, although more convenient for the patient, may reduce accuracy through inadequate bladder emptying, but a good compromise is to make a collection overnight. As long as the bladder was emptied at the beginning of the test (before retiring) and when the final collection was made (on rising), the urine production rate can be calculated and substituted in the It is essential that a reliable method is employed to measure plasma and urine creatinine concentrations. Colorimetric assays tend to overestimate creatinine since they detect non-creatinine chromogens. But even when reliable methods are used, it should be appreciated that the creatinine clearance is based on four measurements: plasma and urine creatinine concentrations, urine volume and time. Each has an inherent inaccuracy and the overall analytical variance will be the sum of the individual variances. The way in which creatinine is handled by the kidney, coupled with the fact that, in any one individual, the rate of production is relatively constant from day-to-day, means that the plasma creatinine concentration alone can be used as an index of renal function. But, although it is widely used, it has a number of disadvantages and these must be borne in mind when interpreting plasma creatinine concentrations. Perhaps the most important becomes apparent from considering the familiar This is of critical importance to clinical practice, since in early renal disease (when it might be supposed that therapeutic intervention would be most beneficial), the plasma creatinine concentration may be ‘normal’ – that is, within the reference range – in spite of the GFR being reduced. Plasma creatinine concentration is thus a relatively insensitive index of mild renal functional impairment. At low values of GFR, however, it becomes extremely sensitive, although its accuracy declines because of the increased contribution of tubular secretion to overall renal excretion. Other factors that must be taken into account in assessing the significance of plasma creatinine concentrations include muscle mass, diet (a recent meal including meat, particularly if stewed, may cause a transient increase in plasma creatinine concentration) and the presence in the plasma of substances that interfere with the assay. Ketones, bilirubin, cephalosporins and spironolactone have all been implicated, depending on the method used, but especially in Jaffé-(picric acid) based assay systems. Enzymatic assays for creatinine are said to be more specific than older colorimetric methods, but they are still prone to interference by certain drugs such as acetylcysteine and phenindione. The importance of muscle mass is exemplified by the changes that occur with age. In healthy individuals, GFR, and thus creatinine clearance, declines with increasing age from about the end of the 4th decade, at a rate of approximately 1 mL/min per year. However, plasma creatinine concentration does not normally rise with age; this is because of a decrease in production rate, thought to reflect the tendency for muscle mass to fall with age. Although comparison of a measured plasma creatinine concentration with a reference range can be misleading, the fact that intraindividual variation is less than interindividual variation means that the detection of a change in creatinine concentration in an individual provides a more sensitive indication of a change in renal function. Nevertheless, the biological and analytical variance (even using the best assays) results in a critical difference of about 17% (20 μmol/L at a plasma creatinine concentration of 120 μmol/L). In patients with advancing renal disease, the progressive loss of renal function with time can be expressed as a graph of reciprocal plasma creatinine concentration against time (since GFR is proportional to 1/[plasma creatinine]). A steady loss of renal function will cause this plot to be rectilinear (Fig. 7.5). Such plots are useful in patients with irreversibly declining renal function to help predict when renal replacement will become necessary, so that, for example appropriate means for vascular access for haemodialysis can be provided. FIGURE 7.5 The progression of chronic kidney disease. Hypothetical curves to show (A) the decline in glomerular filtration rate (GFR); (B) the increase in plasma creatinine concentration and (C) the decrease in reciprocal plasma creatinine concentration. The broken lines show the changes that might be expected if the decline in function was to accelerate for any reason (see Box 7.6).
The kidneys, renal function and kidney disease
ANATOMY
Gross anatomy
Microstructure
The glomerulus
The proximal convoluted tubule
The loop of Henle
The distal convoluted tubule and collecting duct
Other specialized cells
Blood vessels
RENAL FUNCTION
Renal blood flow and its control
Glomerular function
Tubular function
The proximal convoluted tubule
The loop of Henle
Tubuloglomerular feedback
The role of urea
The distal convoluted tubule
The collecting duct
Diuresis
RENAL DISEASE AND ITS PRESENTATION
Introduction
Manifestations of renal disease
Diseases affecting the kidneys
THE ASSESSMENT OF RENAL FUNCTION
Introduction
Biochemical tests of renal function
Urinalysis
Appearance
Specific gravity and osmolality
pH
Glucose
Protein
Urinary sediment
Other substances
Measurement of glomerular filtration rate
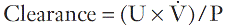
![]() is the rate of formation of urine and P the plasma concentration of the substance. It should be noted that
is the rate of formation of urine and P the plasma concentration of the substance. It should be noted that ![]() is a rate; it has the dimensions (volume/time) and its determination requires the collection and measurement of the total amount of urine formed over a known period of time. The units of the various quantities are usually adjusted so that the clearance is expressed as mL/min.
is a rate; it has the dimensions (volume/time) and its determination requires the collection and measurement of the total amount of urine formed over a known period of time. The units of the various quantities are usually adjusted so that the clearance is expressed as mL/min.

Inulin clearance
Creatinine clearance
![]() formula.
formula.
Plasma creatinine concentration
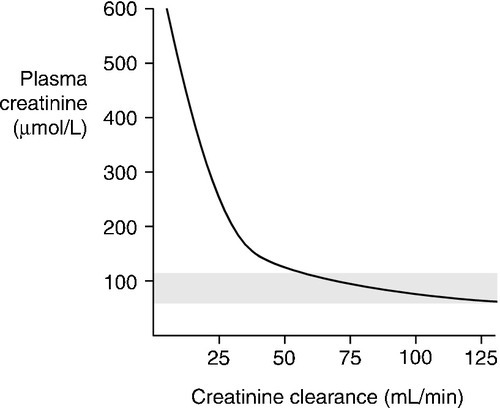
![]() formula for deriving the GFR. Plasma creatinine concentration is inversely related to GFR, not directly related. This means that a plot of plasma creatinine concentration against GFR has the form of a hyperbola (Fig. 7.4). At any concentration of creatinine, halving the GFR will double the plasma creatinine concentration. But given the wide reference range for plasma creatinine concentration in the healthy population, this means that in an individual, the plasma creatinine concentration could be within the reference limits, yet the GFR be only one-half of normal.
formula for deriving the GFR. Plasma creatinine concentration is inversely related to GFR, not directly related. This means that a plot of plasma creatinine concentration against GFR has the form of a hyperbola (Fig. 7.4). At any concentration of creatinine, halving the GFR will double the plasma creatinine concentration. But given the wide reference range for plasma creatinine concentration in the healthy population, this means that in an individual, the plasma creatinine concentration could be within the reference limits, yet the GFR be only one-half of normal.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree