5-Hydroxytryptamine (Serotonin) and Dopamine
5-Hydroxytryptamine (5HT, serotonin) and dopamine (DA) have prominent actions in the CNS and the periphery. Fourteen 5HT receptor subtypes and five DA receptor subtypes have been delineated by pharmacological analyses and cDNA cloning. The availability of cloned receptors has allowed the development of subtype-selective drugs and the elucidation of actions of these neurotransmitters at a molecular level.
5-HYDROXYTRYPTAMINE
5HT is found in high concentrations in enterochromaffin cells throughout the GI tract, in storage granules in platelets, and broadly throughout the CNS. 5HT regulates smooth muscle in the cardiovascular system and the GI tract and enhances platelet aggregation.
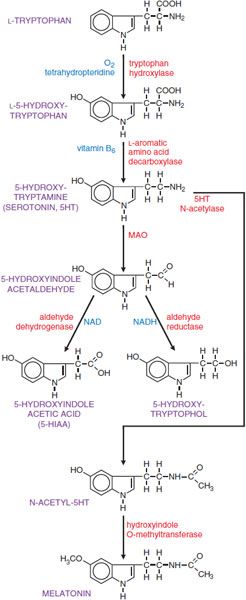
SYNTHESIS AND METABOLISM OF 5HT. 5HT is synthesized by a 2-step pathway from the tryptophan (Figure 13–1).
Figure 13–1 Synthesis and inactivation of serotonin. Enzymes are identified in red lettering, and cofactors are shown in blue.
Tryptophan is actively transported into the brain by a carrier protein. Levels of tryptophan in the brain reflect its plasma concentration and the plasma concentrations of amino acids that compete for the same transporter. Tryptophan hydroxylase, the rate-limiting enzyme in the synthetic pathway, converts tryptophan to L-5-hydroxytryptophan; the enzyme is not regulated by end-product inhibition. Brain tryptophan hydroxylase is not generally saturated with substrate; consequently, the concentration of tryptophan in the brain influences the synthesis of 5HT.
Aromatic l-amino acid decarboxylase (AADC) converts L-5-hydroxytryptophan to 5HT; it is widely distributed and has broad substrate specificity. The synthesized product, 5HT, is accumulated in secretory granules by a vesicular monoamine transporter (VMAT2); vesicular 5HT is released by exocytosis from serotonergic neurons. In the nervous system, the action of released 5HT is terminated via neuronal uptake by a specific 5HT transporter (SERT), localized in the membrane of serotonergic axon terminals and in the membrane of platelets. This uptake system is the means by which platelets acquire 5HT, since they lack the enzymes required for 5HT synthesis. The amine transporters are distinct from VMAT2, which concentrates amines in intracellular storage vesicles and is a nonspecific amine carrier, whereas the 5HT transporter is specific.
The principal route of metabolism of 5HT involves oxidative deamination by monoamine oxidase (MAO); the aldehyde intermediate thus formed is converted to 5-hydroxyindole acetic acid (5-HIAA) by aldehyde dehydrogenase (see Figure 13–1). 5-HIAA is actively transported out of the brain by a process that is sensitive to the nonspecific transport inhibitor, probenecid. 5-HIAA from brain and peripheral sites of 5HT storage and metabolism is excreted in the urine along with small amounts of 5-hydroxytryptophol sulfate or glucuronide conjugates. The usual range of urinary excretion of 5-HIAA by a normal adult is 2-10 mg daily. Ingestion of ethanol results in elevated amounts of NADH2 (see Chapter 23), which diverts 5-hydroxyindole acetaldehyde from the oxidative route to the reductive pathway and tends to increase the excretion of 5-hydroxytryptophol and correspondingly reduce the excretion of 5-HIAA.
Of the 2 isoforms of monoamine oxidase (MAO; see Chapter 8), MAO-A preferentially metabolizes 5HT and NE. Dopamine and tryptamine are metabolized equally well by both isoforms. Neurons contain both isoforms of MAO, localized primarily in the outer membrane of mitochondria. MAO-B is the principal isoform in platelets, which contain large amounts of 5HT.
PHYSIOLOGICAL FUNCTIONS OF SEROTONIN
MULTIPLE 5HT RECEPTORS
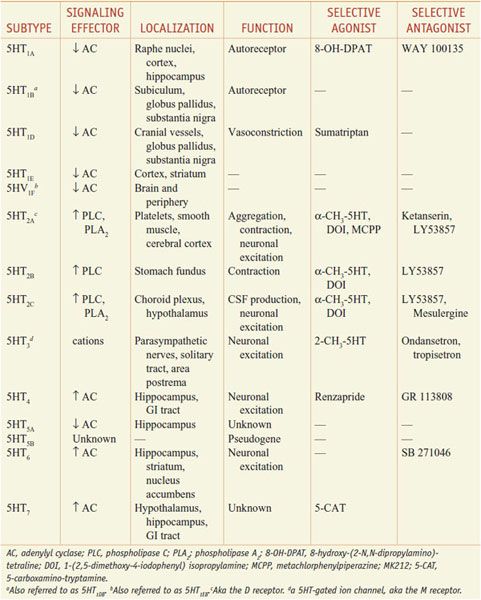
The multiple 5HT receptor subtypes cloned comprise the largest known neurotransmitter-receptor family. The 5HT receptor subtypes are expressed in distinct but often overlapping patterns and are coupled to different transmembrane-signaling mechanisms (Table 13–1).
Table 13–1
Serotonin Receptor Subtypes
Four of seven 5HT receptor families have defined functions. The 5HT1, 5HT2, and 5HT4–7 receptor families are members of the superfamily of GPCRs.
• The 5HT1-receptor subfamily consists of 5 members, all of which preferentially couple to Gi/o and inhibit adenylyl cyclase.
• The 3 subtypes of 5HT2 receptors couple to Gq/G11 proteins and activate the PLC-DAG/IP3-Ca2+-PKC pathway. 5HT2A and 5HT2C receptors also activate phospholipase A2, promoting the release of arachidonic acid.
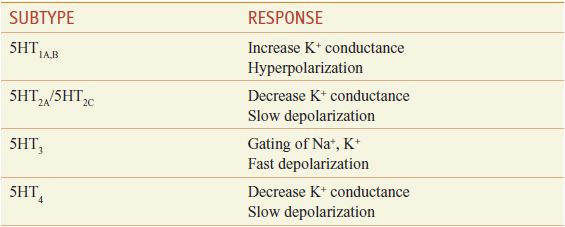
• The 5HT3 receptor is the only monoamine neurotransmitter receptor that functions as a ligand-operated ion channel. Activation of 5HT3 receptors elicits a rapidly desensitizing depolarization, mediated by the gating of cations.
• 5HT4 receptors couple to Gs to activate adenylyl cyclase and increase intracellular cyclic AMP.
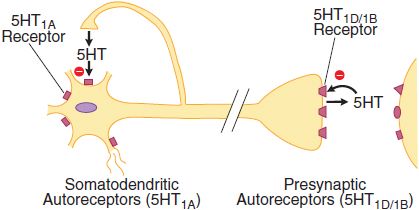
The 5HT1A, 5HT1B, and 5HT1D receptor subtypes also activate a receptor-operated K+ channel and inhibit a voltage-gated Ca2+ channel. The 5HT1A receptor is found in the raphe nuclei of the brainstem, where it functions as an inhibitory somatodendritic autoreceptor on cell bodies of serotonergic neurons (Figure 13–2). The 5HT1D/1B receptor functions as an autoreceptor on axon terminals, inhibiting 5HT release. 5HT1D receptors, abundantly expressed in the substantia nigra and basal ganglia, regulate the firing rate of DA-containing cells and the release of DA at axonal terminals.
Figure 13–2 Two classes of 5HT autoreceptors with differential localizations. Somatodendritic 5HT1A autoreceptors decrease raphe cell firing when activated by 5HT released from axon collaterals of the same or adjacent neurons. The receptor subtype of the presynaptic autoreceptor on axon terminals in the forebrain has different pharmacological properties and has been classified as 5HT1D (in humans) or 5HT1B (in rodents). This receptor modulates the release of 5HT. Postsynaptic 5HT1 receptors are also indicated.
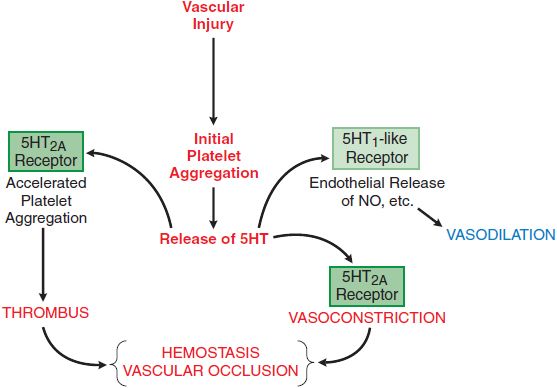
5HT2A receptors are broadly distributed in the CNS (primarily in serotonergic terminal areas, with high densities in prefrontal and parietal areas, and somatosensory cortex), as well as in blood platelets (Figure 13–3) and smooth muscle cells. The 5HT2C receptor has been implicated in the control of cerebrospinal fluid production and in feeding behavior and mood.
Figure 13–3 The local influences of platelet 5HT. The release of 5HT stored in platelets is triggered by aggregation. The local actions of 5HT include feedback actions on platelets (shape change and accelerated aggregation) mediated by interaction with platelet 5HT2A receptors, stimulation of NO production mediated by 5HT1-like receptors on vascular endothelium, and contraction of vascular smooth muscle mediated by 5HT2A receptors. These influences act in concert with many other mediators that are not shown to promote thrombus formation and hemostasis. See Chapter 30 for details of adhesion and aggregation of platelets and factors contributing to thrombus formation and blood clotting.
5HT3 receptors are located on parasympathetic terminals in the GI tract, including vagal and splanchnic afferents. In the CNS, a high density of 5HT3 receptors occurs in the solitary tract nucleus and the area postrema. 5HT3 receptors in both the GI tract and the CNS participate in the emetic response, providing a basis for the antiemetic property of 5HT3 receptor antagonists.
In the CNS, 5HT4 receptors are found on neurons of the superior and inferior colliculi and in the hippocampus. In the GI tract, 5HT4 receptors are located on neurons of the myenteric plexus and on smooth muscle and secretory cells. In the GI tract, stimulation of the 5HT4 receptor is thought to evoke secretion and to facilitate the peristaltic reflex. The latter effect may explain the utility of prokinetic benzamides in GI disorders (see Chapter 46).
Two subtypes of the 5HT5 receptor have been cloned; although the 5HT5A receptor has been shown to inhibit adenylyl cyclase, functional coupling of the cloned 5HT5B receptor has not yet been described. Two other cloned receptors, 5HT6 and 5HT7, are linked to activation of adenylyl cyclase. 5HT7 receptors may play a role in the relaxation of smooth muscle in the GI tract and the vasculature. The atypical antipsychotic drug clozapine has a high affinity for 5HT6 and 5HT7 receptors; whether this property is related to the broader effectiveness of clozapine compared to conventional antipsychotic drugs is not known (see Chapter 16).
ACTIONS OF 5HT IN PHYSIOLOGICAL SYSTEMS
PLATELETS. Platelets differ from other formed elements of blood in expressing mechanisms for uptake, storage, and endocytotic release of 5HT. 5HT is not synthesized in platelets, but is taken up from the circulation and stored in secretory granules by active transport, similar to the uptake and storage of serotonin by serotonergic nerve terminals.
Thus, Na+-dependent transport across the platelet plasma membrane via the 5HT transporter is followed by VMAT2-mediated uptake into storage granules creating a gradient of 5HT as high as 1000:1 with an internal concentration of 0.6 M in the storage vesicles.
When platelets make contact with injured endothelium (see Chapter 30), they release substances that promote platelet aggregation, and secondarily, they release 5HT (see Figure 13–3). 5HT binds to platelet 5HT2A receptors and elicits a weak aggregation response that is markedly augmented by the presence of collagen. If the damaged blood vessel is injured to a depth where vascular smooth muscle is exposed, 5HT exerts a direct vasoconstrictor effect, thereby contributing to hemostasis, which is enhanced by locally released autocoids (thromboxane A2, kinins, and vasoactive peptides). Conversely, 5HT may interact with endothelial cells to stimulate production of NO and antagonize its own vasoconstrictor action, as well as the vasoconstriction produced by other locally released agents.
CARDIOVASCULAR SYSTEM. The classical response of blood vessels to 5HT is contraction, particularly in the splanchnic, renal, pulmonary, and cerebral vasculatures. 5HT also induces a variety of responses by the heart that are the result of activation of multiple 5HT receptor subtypes, stimulation or inhibition of autonomic nerve activity, or dominance of reflex responses to 5HT.
Thus, 5HT has positive inotropic and chronotropic actions on the heart that may be blunted by simultaneous stimulation of afferent nerves from baroreceptors and chemoreceptors. Activation of 5HT3 receptors on vagus nerve endings elicits the Bezold-Jarisch reflex, causing extreme bradycardia and hypotension. The local response of arterial blood vessels to 5HT also may be inhibitory, the result of the stimulation of endothelial NO production and prostaglandin synthesis and blockade of NE release from sympathetic nerves. Conversely, 5HT amplifies the local constrictor actions of NE, ANGII, and histamine, which reinforce the hemostatic response to 5HT.
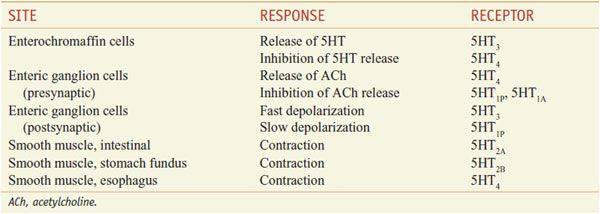
GI TRACT. Enterochromaffin cells in the gastric mucosa are the site of the synthesis and most of the storage of 5HT in the body and are the source of circulating 5HT. Motility of gastric and intestinal smooth muscle may be either enhanced or inhibited by at least 6 subtypes of 5HT receptors (Table 13–2).
Table 13–2
Some Actions of 5HT in the Gastrointestinal Tract
Basal release of enteric 5HT is augmented by mechanical stretching, such as that caused by food, and by efferent vagal stimulation. Released 5HT enters the portal vein and is subsequently metabolized by MAO-A in the liver. 5HT that survives hepatic oxidation may be captured by platelets or rapidly removed by the endothelium of lung capillaries and inactivated. 5HT released from enterochromaffin cells also acts locally to regulate GI function. Abundant 5HT3 receptors on vagal and other afferent neurons and on enterochromaffin cells play a pivotal role in emesis (see Chapter 46). Enteric 5HT triggers peristaltic contraction when released in response to acetylcholine, sympathetic nerve stimulation, increases in intraluminal pressure, and lowered pH.
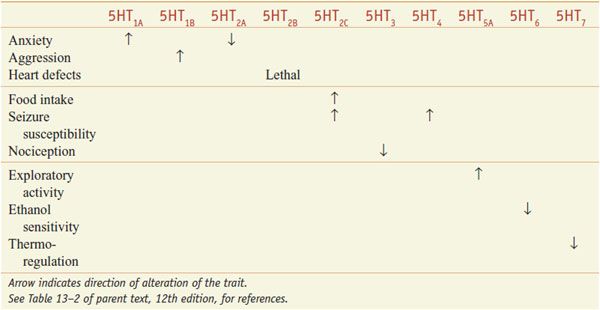
CNS. All of the cloned 5HT receptors are expressed in the brain; 5HT influences a multitude of brain functions, including sleep, cognition, sensory perception, motor activity, temperature regulation, nociception, mood, appetite, sexual behavior, and hormone secretion. The roles of specific 5HT receptors in these functions have been defined in receptor knockout mice (Table 13–3).
Table 13–3
Physiological Roles of 5HT Receptors Defined by Phenotypes in Knockout Mice
The principal cell bodies of 5HT neurons are located in raphe nuclei of the brainstem and project throughout the brain and spinal cord (see Chapter 14). In addition to being released at discrete synapses, release of serotonin also seems to occur at sites of axonal varicosities that do not form distinct synaptic contacts. 5HT released at nonsynaptic varicosities is thought to diffuse to outlying targets, rather than acting on discrete synaptic targets, perhaps acting as a neuromodulator as well as a neurotransmitter (see Chapter 14). Serotonergic nerve terminals contain the proteins needed to synthesize 5HT from L-tryptophan. Newly formed 5HT is rapidly accumulated in synaptic vesicles (through VMAT2), where it is protected from MAO. 5HT released by nerve-impulse flow is reaccumulated into the presynaptic terminal by the 5HT transporter, SERT (SLC6A4; see Chapter 5). Presynaptic reuptake is a highly efficient mechanism for terminating the action of 5HT released by nerve-impulse flow. MAO localized in postsynaptic elements and surrounding cells rapidly inactivates 5HT that escapes neuronal reuptake and storage.
ELECTROPHYSIOLOGY. The physiological consequences of 5HT release vary with the brain area and the neuronal element involved, as well as with the 5HT receptor subtype(s) expressed (Table 13–4).
Table 13–4
Electrophysiological Effects of 5HT Receptors
BEHAVIOR
SLEEP-WAKE CYCLE. 5HT plays a role in sleep-wake cycle.
Depletion of 5HT with p-Chlorophenylalanine, a tryptophan hydroxylase inhibitor, elicits insomnia that is reversed by the 5HT precursor, 5-hydroxytryptophan. Conversely, treatment with L-tryptophan or with nonselective 5HT agonists accelerates sleep onset and prolongs total sleep time. 5HT antagonists reportedly can increase and decrease slow-wave sleep, probably reflecting interacting or opposing roles for subtypes of 5HT receptors. One relatively consistent finding in humans and in laboratory animals is an increase in slow-wave sleep following administration of a selective 5HT2A/2C-receptor antagonist such as ritanserin.
AGGRESSION AND IMPULSIVITY. 5HT serves a critical role in aggression and impulsivity.
Human studies reveal a correlation between low cerebrospinal fluid 5-HIAA and violent impulsivity and aggression. Knockout mice lacking the 5HT1B receptor exhibit extreme aggression, suggesting either a role for 5HT1B receptors in the development of neuronal pathways important in aggression or a direct role in the mediation of aggressive behavior. A human genetic study identified a point mutation in the gene encoding MAO-A, which was associated with extreme aggressiveness and mental retardation; this has been confirmed in knockout mice lacking MAO-A.
ANXIETY AND DEPRESSION. The effects of 5HT–active drugs in anxiety and depressive disorders, like the effects of selective serotonin reuptake inhibitors (SSRIs), strongly suggest a role for 5HT in the neurochemical mediation of these disorders.
Inhibition of neuronal reuptake of 5HT via the transporter SERT (SLC6A4) prolongs the dwell-time of 5HT in the synapse. SSRIs, such as fluoxetine (PROZAC, others), potentiate and prolong the action of 5HT released by neuronal activity. When coadministered with L-5-hydroxytryptophan, SSRIs elicit a profound activation of serotonergic responses. SSRIs (citalopram [CELEXA], escitalopram [LEXAPRO], fluoxetine, fluvoxamine, paroxetine [PAXIL], and sertraline [ZOLOFT]) are the most widely used treatment for endogenous depression (see Chapter 15).
APPETITE. Sibutramine (MERIDIA), an inhibitor of the reuptake of 5HT, NE, and DA, is used as an appetite suppressant in the management of obesity.
Sibutramine is classified as a selective serotonin-norepinephrine reuptake inhibitor (SNRI). Other SNRIs include duloxetine (CYMBALTA; approved for depression, anxiety, peripheral neuropathy, and fibromyalgia), venlafaxine (EFFEXOR; approved for the treatment of depression, anxiety, and panic disorders), desvenlafaxine (PRISTIQ; approved for depression), and milnacipran (SAVELLA; approved for fibromyalgia).
5HT RECEPTOR AGONISTS AND ANTAGONISTS
5HT RECEPTOR AGONISTS
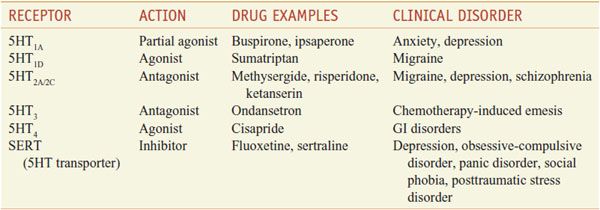
Direct-acting 5HT receptor agonists have widely different chemical structures, as well as diverse pharmacological properties and are used in the pharmacotherapy of migraine, anxiety, depression, chemotherapy-induced emesis, and disorders of GI motility (Table 13–5).
Table 13–5
Serotonergic Drugs: Primary Actions and Clinical Indications
5HT RECEPTOR AGONISTS AND MIGRAINE. 5HT seems to be a key mediator in the pathogenesis of migraine. Consistent with the 5HT hypothesis of migraine, 5HT receptor agonists are a mainstay for acute treatment of migraine headaches. The efficacy of antimigraine drugs varies with the absence or presence of aura, duration of the headache, its severity and intensity, and as yet undefined environmental and genetic factors.
5HT1B/1D RECEPTOR AGONISTS: THE TRIPTANS. The triptans are indole derivatives that are effective, acute antimigraine agents. Their capacity to decrease the nausea and vomiting of migraine is an important advance in the treatment of the condition. Available compounds include almotriptan (AXERT), eletriptan (RELPAX), frovatriptan (FROVA), naratriptan (AMERGE), rizatriptan (MAXALT, others), sumatriptan (IMITREX, others), and zolmitriptan (ZOMIG). Sumatriptan for migraine headaches is also marketed in a fixed-dose combination with naproxen (TREXIMET).
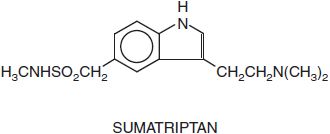
Pharmacological Properties. The pharmacological effects of the triptans appear to be limited to the 5HT1 family of receptors, providing evidence that this receptor subclass plays an important role in the acute relief of a migraine attack. The triptans interact potently with 5HT1B
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree