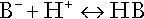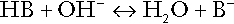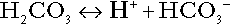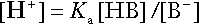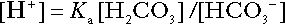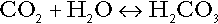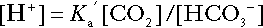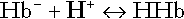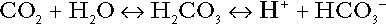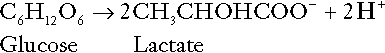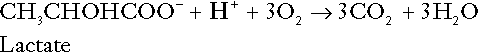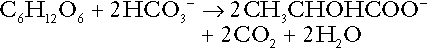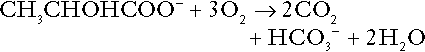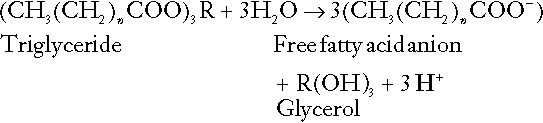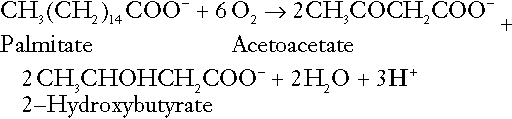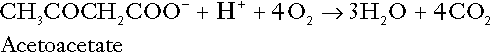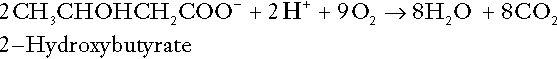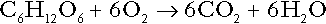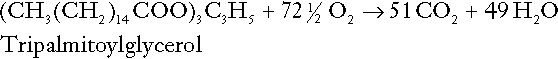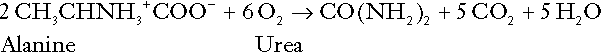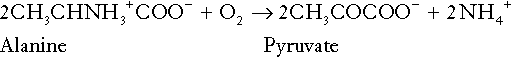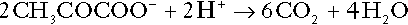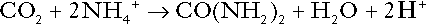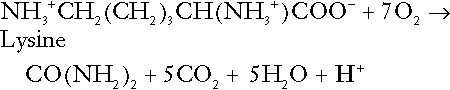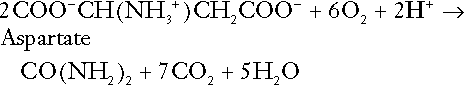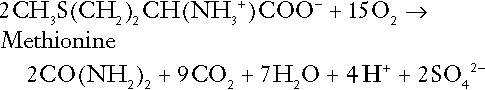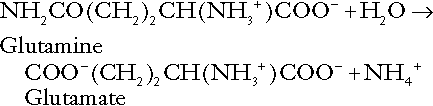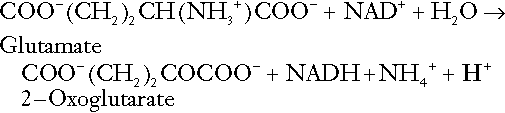CHAPTER 5 CHAPTER OUTLINE THE PHYSIOLOGICAL ROLE OF HYDROGEN IONS THE ASSESSMENT OF ACID–BASE STATUS DISORDERS OF HYDROGEN ION HOMOEOSTASIS The interpretation of acid–base data Mixed disorders of hydrogen ion homoeostasis The role of haemoglobin in oxygen transport The effects of pulmonary disease on oxygen uptake into blood Disorders of both hydrogen ion homoeostasis and tissue oxygenation will be discussed in this chapter. Abnormalities of hydrogen ion homoeostasis occur frequently in respiratory disorders, as a result of changes in the rate of excretion of carbon dioxide. Such disorders can also affect oxygenation, and impaired tissue oxygenation is an important potential cause of acidosis. Furthermore, hydrogen ion concentration, carbon dioxide and oxygen are measured using related technologies, usually with the same instrument. The first part of this chapter deals with hydrogen ion homoeostasis and its disorders (colloquially often referred to as ‘acid–base balance’ and ‘acid–base disorders’, respectively), while the second part deals with the mechanism whereby oxygen is made available to the tissues, disorders in which tissue oxygenation is impaired and how tissue oxygenation is measured. Hydrogen ions are ubiquitous in the body and maintenance of appropriate concentrations is critical to normal function. The gradient of hydrogen ion concentration between the inner and outer mitochondrial membrane drives oxidative phosphorylation; changes in hydrogen ion concentration can affect the surface charge and physical conformation of proteins and thus their function, and hydrogen ion concentration determines the degree of ionization of weak acids and bases and can thus affect the disposition of such substances, among which are many with important physiological and pharmacological functions. The hydrogen ion concentration of the blood is normally controlled within narrow limits, in health rarely exceeding 46 nmol/L or falling below 35 nmol/L. This regulation is achieved in spite of the continuous production of hydrogen ions as a result of the normal processes of metabolism. Intracellular hydrogen ion concentration is, in general, higher; in the cytosol being slightly so while in lysosomes it is several orders of magnitude higher. However, the interior of mitochondria is slightly alkaline. An increase in the hydrogen ion concentration of the blood is termed acidaemia and a decrease, alkalaemia. The term ‘acidosis’ strictly describes a pathological disturbance that can result in acidaemia, but may not necessarily do so because of the simultaneous existence of another disturbance (possibly the result of a physiological compensatory process) that has an opposing effect. Similarly, alkalaemia is not always present in alkalosis. These distinctions, although made much of by some authors, often only introduce confusion and will not be pursued in this chapter. Strictly speaking, it is the activity of hydrogen ions and not their concentration that is relevant, and devices for measuring hydrogen ions respond to their activity. Activity and concentration are only the same in ideal solutions, which biological fluids are not, but the distinction can be ignored for practical purposes. Hydrogen ion concentration can also be expressed in terms of pH. The pH of a solution is the logarithm (base 10) of the reciprocal of hydrogen ion concentration (or minus the logarithm of hydrogen ion concentration). Thus, a solution with hydrogen ion concentration of 100 nmol/L (100 × 10− 9 mol/L) has a pH of 7.00 (log10 1/100 × 10− 9). pH does not have units; it varies in a reciprocal and nonlinear fashion with hydrogen ion concentration. The reference range for the pH of blood, corresponding to the range for hydrogen ion concentration given above, is 7.36–7.42. It is necessary, in discussing hydrogen ion homoeostasis and its disorders, to consider the production and disposal of hydrogen ions; it is therefore logical to discuss the effects of changes in these processes on hydrogen ion concentration rather than on a derived unit. Furthermore, as will be seen, the analysis of disorders of hydrogen ion homoeostasis is facilitated considerably if direct measurements are used. In health, the rates of formation of hydrogen ions and of their consumption and excretion are in balance, but disturbances of hydrogen ion homoeostasis occur frequently and in a wide variety of disease states. They are classified traditionally as respiratory or non-respiratory in origin, according to whether the primary abnormality is the result of an excess or deficiency of carbon dioxide (respiratory) or of bicarbonate (non-respiratory). Non-respiratory disturbances are often referred to as metabolic. As will be seen, however, a respiratory disorder that causes hypoxia may produce an acid–base abnormality whose characteristics are non-respiratory, or even combine respiratory and non-respiratory features. Nevertheless, the distinction is a useful one and is of considerable value in analysing disorders of hydrogen ion homoeostasis. What follows is a brief, simplified account of the essential features of buffering, sufficient for an appreciation of the physiology of hydrogen ion homoeostasis. In essence, buffer systems limit the extent to which the hydrogen ion concentration actually changes in the face of any tendency to change. A buffer system (or buffer pair) consists of a weak (that is, only partly dissociated) acid and its conjugate base (that is, the anion that combines with a hydrogen ion to form the acid). If the acid is HB and the conjugate base is B−, the relevant reactions are: and An example of central importance to physiology is the carbonic acid–bicarbonate system. Carbonic acid is a weak acid, which partly dissociates to bicarbonate and hydrogen ion: If an amount of hydrogen ions were to be added to a solution containing this buffer pair, some would combine with bicarbonate to form carbonic acid, thus limiting the increase in hydrogen ion concentration that otherwise would be expected to occur, but at the expense of consuming bicarbonate. Removal of hydrogen ions (or addition of hydroxyl, which, by combining with hydrogen ions to form water, has an identical effect) would cause dissociation of carbonic acid, producing hydrogen ions (and bicarbonate) and thus limiting the fall in hydrogen ion concentration that otherwise would be expected to occur. It will be apparent that if a buffer is to function effectively in the face of equal tendencies for hydrogen ion concentration to increase and decrease, the concentrations of the acid and conjugate base should be equal, for example for the acid HB, [HB] = [B−]. Since at equilibrium: (where Ka is the equilibrium constant), it follows that a buffer will be most effective when the hydrogen ion concentration to be defended is numerically similar to the equilibrium constant. It should also be appreciated that hydrogen ion concentration, [H+], depends not on the absolute values for the concentrations of acid and conjugate base, but on the ratio of their concentrations. On the other hand, the buffer capacity, that is, the extent to which the buffer can absorb hydrogen ions, clearly will depend upon the absolute values of these concentrations. For bicarbonate, it follows from the general equation (Eqn. 4) that: The concentration of carbonic acid cannot readily be determined, but it is directly proportional to the concentration of carbon dioxide [CO2], since carbonic acid is formed by the hydration of carbon dioxide: Thus, Equation 5 can be rewritten in the form: where Ka′ is a constant numerically incorporating the equilibrium constants for the reactions represented by Equations 3 and 5. The value of Ka′ is approximately 800 nmol/L and, at physiological extracellular hydrogen ion concentration (40 nmol/L), the numerical value of the molar ratio In practice, carbon dioxide concentration cannot be readily determined, but it is related to the partial pressure, PCO2, such that [CO2] = 0.225 × PCO2 when PCO2 is measured in kilopascals (kPa). The monohydrogen and dihydrogen phosphate ions ( All proteins can buffer hydrogen ions to some extent by virtue of their content of polar amino acid residues. Haemoglobin (Hb) is an important buffer. The reaction can be represented as: although each haemoglobin molecule is capable of buffering a number of hydrogen ions. It is relevant to note that haemoglobin is a more effective buffer for hydrogen ions when it is in the deoxygenated rather than the oxygenated form, and that oxygen release is facilitated by the buffering (the Bohr effect). The efficacy of haemoglobin as a buffer is enhanced by the presence in erythrocytes of the enzyme carbonate dehydratase, which catalyses the hydration of carbon dioxide (Eqn. 6). Despite the fact that they are responsible for the majority of oxygen transport in the blood, erythrocytes obtain their energy anaerobically, by glycolysis, and thus do not generate carbon dioxide. In the capillary beds of tissues, carbon dioxide produced by aerobic metabolism readily diffuses down the concentration gradient into erythrocytes, where it is hydrated to form carbonic acid, which dissociates to form bicarbonate and hydrogen ions (Fig. 5.1). The latter are buffered by haemoglobin while bicarbonate diffuses out of the cells in exchange for chloride, so that the products of the dissociation of carbonic acid are removed, allowing further dissociation to occur and thus, by a mass action effect, stimulating its formation. In the lungs, alveolar PCO2 is lower than venous PCO2 and the process reverses, carbon dioxide being generated and excreted, while the release of hydrogen ions from haemoglobin favours oxygen uptake. FIGURE 5.1 Transport and buffering of carbon dioxide in erythrocytes. Carbon dioxide is converted into carbonic acid in erythrocytes; this dissociates to form bicarbonate, which diffuses into the plasma in exchange for chloride and hydrogen ions, which are buffered by haemoglobin. In the alveoli, the reverse process liberates carbon dioxide. Thus, in addition to transporting carbon dioxide, the conversion of carbon dioxide to bicarbonate serves to minimize the potential change in the value of the ratio Although plasma proteins buffer hydrogen ions, their molar concentrations are lower than that of haemoglobin and their buffering capacity is less. In contrast, intracellular and tissue proteins make an important contribution to buffering. In chronic acidosis, they may contribute up to one-third of total buffer capacity. Buffering by bone is particularly important. It is often stated that ammonia is an important buffer in the urine because it can combine with hydrogen ions to form ammonium ions. This cannot be true. Ammonium is a very weak acid, with an equilibrium constant approximately 100 times lower than physiological hydrogen ion concentration, so that when ammonia is produced in the body, it is immediately and almost completely converted to ammonium ions. The urinary excretion of ammonium is of relevance to hydrogen ion homoeostasis because it represents a route for the disposal of ammonium which, unlike urea synthesis, does not result in the generation of hydrogen ions (see p. 70). The fact that, on a normal diet, the daily excretion of hydrogen ions by the kidneys (the only physiologically important route of excretion) is 40–80 mmol tends to distract from the fact that there is a massive endogenous turnover of hydrogen ions. In the resting adult, intermediary metabolism accounts for a hydrogen ion turnover of 2500–3000 mmol/24 h (Table 5.1). Normally, the rates of hydrogen ion formation and utilization during intermediary metabolism are in overall balance, although any discrepancy can have a major effect on hydrogen ion concentration. But even this turnover of hydrogen ions appears insignificant in comparison with that which is associated with the turnover of adenine nucleotides – the movement of hydrogen ions that takes place across the mitochondrial membrane during oxidative phosphorylation and the synthesis and hydrolysis of ATP. This has been estimated at 500 mol/24 h. The potential for a disturbance in these processes to cause an acidosis is clearly colossal, although, in health, the rates of adenine nucleotide reduction and oxidation and of ATP formation and utilization are equal, so that these processes have no net effect on hydrogen ion homoeostasis. This may not, however, be true in disease. As a result of oxidative metabolism, carbon dioxide is produced and excreted by the lungs. The rates of formation and excretion are normally equal, but carbon dioxide can combine with water to form carbonic acid, and the daily production of carbon dioxide in a resting adult is a potential source of approximately 15–20 mol of hydrogen ions. As has been alluded to, disorders affecting the excretion of carbon dioxide are an important cause of abnormalities of hydrogen ion homoeostasis. Tendencies for hydrogen ion concentration to change can be limited to some extent by buffering, but this process can only offer a temporary solution to an imbalance between the rates of hydrogen ion production and disposal, because the body’s buffers have a limited capacity. Physiological processes often bring about a partial reversal of a change in hydrogen ion concentration (see Compensation, below), but ultimate correction of any disturbance requires equalization of the rates of production and disposal of hydrogen ion. The processes involved in hydrogen ion production and utilization are summarized in Table 5.1. They comprise: processes involving carbon dioxide formation, reactions of intermediary metabolism and processes involving ‘fixed’ acids. In addition, and responsible for the bulk of hydrogen ion turnover, are the reactions involved in the complete oxidation of energy substrates. These processes are interlinked, but it is instructive to consider them separately. The role of carbon dioxide in relation to the formation of hydrogen ions has been mentioned above. Carbon dioxide, produced by oxidative metabolism, can become hydrated to carbonic acid, a weak acid that partly dissociates to hydrogen ion and bicarbonate: The equilibrium for this reaction strongly favours carbon dioxide and water, but in tissues containing carbonate dehydratase (e.g. tubular cells in the kidneys), the rate of formation of carbonic acid is increased and it can become an important source of bicarbonate and hydrogen ions. The most familiar process of intermediary metabolism that results in the formation of hydrogen ions is anaerobic glycolysis, the metabolism of glucose to lactate. The overall equation for this reaction is: This process, which takes place particularly in skeletal muscle and erythrocytes, results in the formation of ~ 1.3 mol of hydrogen ions per 24 h in a 70 kg man at rest. The major route of disposal of lactate is glucose synthesis by gluconeogenesis in the liver and kidneys. The overall equation for this process is the reverse of that for glycolysis (most enzymes involved being common to both pathways, although some are unique). Gluconeogenesis thus consumes hydrogen ions. In health, lactate production and disposal are equal and so, too, are the production and disposal of hydrogen ions by these pathways. At rest, most of the lactate produced is converted back to glucose; during exercise, when lactate production is increased, 50% or more is completely oxidized instead. This process also consumes the hydrogen ions that are generated in its production and results in the formation of carbon dioxide and water: Thus, when lactate production and utilization are in balance, there is no net production of hydrogen ions. However, in disease states, an imbalance between these processes can be responsible for the development of acidosis. The hydrogen ions generated in lactate production will be buffered principally by bicarbonate, so that Equation 10 could be written: Clearly, the reverse reaction, gluconeogenesis, will regenerate the bicarbonate, and Equation 11 can be rewritten to show that this also occurs with complete oxidation of lactate: It is pertinent to point out that hyperlactataemia does not automatically indicate an acidosis. If it is a result of increased lactate production or decreased excretion, acidosis will only be present if the hydrogen ions produced simultaneously are not removed by excretion or metabolism. Intravenous fluids containing sodium lactate (e.g. Hartmann’s solution) are actually a source of alkali, because the lactate ions are metabolized to bicarbonate (Eqn. 13). The liberation of free fatty acids from triglyceride (triacylglycerol) in adipose tissue results in the generation of hydrogen ions. The process is exemplified by the equation: In the liver and adipose tissue, free fatty acids can be re-esterified to triacylglycerol, a process that consumes three hydrogen ions for each molecule of triacylglycerol synthesized. The further metabolism of free fatty acids to ketones in the liver (ketogenesis) also results in hydrogen ion production. An example of the reaction, starting from palmitate, is: In health, this process may account for up to 0.4 mol hydrogen ion per 24 h, although in pathological states, its contribution may be much greater. However, ketoacids (strictly, oxoacids) are utilized as energy sources by skeletal muscle and other tissues. Their oxidation consumes hydrogen ions so that, in health, the overall rates of hydrogen ion production and disposal are equal: In disease, notably in diabetic ketoacidosis, excessive ketogenesis is an important cause of acidosis. Ketonuria may exacerbate the acidosis: ketoacid anions are a potential source of bicarbonate so that their loss in urine (in maximally acidic urine, about half the ketoacids are present in this form) effectively reduces the potential for bicarbonate generation. Glucose can also be completely oxidized to carbon dioxide and water; indeed, this is the major route of glucose metabolism in the body. This is a complex process: oxidation is indirect, involving the transfer of hydrogen ions to adenine nucleotides, which are mainly oxidized by the mitochondrial electron transport system. Electrons are transferred to oxygen and combination with hydrogen ions produces water, while the energy released is transferred to ATP. But as Equation 18 indicates, the complete oxidation of glucose to carbon dioxide does not give rise to net formation of hydrogen ions: the products of its oxidation are carbon dioxide and water. The same is true for the complete oxidation of triacylglycerols: As with glucose oxidation, the process is far more complicated than appears from Equation 19, again involving the reduction of nucleotides, transfer of electrons to molecular oxygen, formation of water and trapping of energy in ATP, but the net production of hydrogen ions is zero. Amino acid metabolism both produces and consumes hydrogen ions, according to the type of amino acid concerned. The metabolism of neutral amino acids eventually results in the formation of urea and carbon dioxide, for example: It is instructive, however, to look at this process in more detail. Most amino acids are metabolized by transamination in the liver to yield the corresponding oxoacid, the amino group being transferred to 2–oxoglutarate to form glutamate. Glutamate undergoes oxidative deamination, the amino group being converted to ammonium. The carbon skeletons of amino acids are in general glucogenic, although some are ketogenic. Ultimately, they will be completely metabolized. Omitting the transamination step, the intermediate stages are: Thus, although urea synthesis generates hydrogen ions, these are utilized during the metabolism of the carbon skeleton so that the metabolism of neutral amino acids does not result in net generation of hydrogen ions provided that the nitrogen is converted into urea. If this does not occur, the metabolism of these amino acids consumes hydrogen ions. The relevance of this is discussed in a later section of this chapter. The complete oxidation of a dibasic amino acid results in the generation of hydrogen ions, for example for lysine: The complete oxidation of a dicarboxylic acid consumes hydrogen ions, for example for aspartate: The complete oxidation of sulphur-containing amino acids (cysteine, methionine) generates hydrogen ions, for example for methionine: In each of these last three examples, it has been assumed that the end-product of the amino nitrogen is urea. If it is not, the effect on hydrogen ion metabolism may be different. Overall, the amino acid composition of dietary protein and the manner of amino acid metabolism is such that, in health, there is a small net production of hydrogen ions. This is disposed of primarily by renal excretion. Carbon dioxide is excreted via the lungs. The respiratory control mechanisms are exquisitely sensitive to carbon dioxide so that, in health (and in the absence of any conscious effort to hypo- or hyperventilate), the rate of carbon dioxide elimination is made equal to the rate of production, and blood carbon dioxide concentration remains constant. Excess hydrogen ions are excreted in the urine and because, overall, the body is a net producer of acid, the urine is usually acidic. But before the urine can be acidified, there must be complete reabsorption of filtered bicarbonate. The glomerular filtrate contains bicarbonate at the same concentration as the plasma. Normally, the urine is virtually bicarbonate-free and, were the filtered bicarbonate not to be reabsorbed, the body’s bicarbonate pool, and thus buffering capacity, would rapidly be depleted. Generation of bicarbonate from carbon dioxide and water necessarily also involves the production of hydrogen ions. The luminal membranes of renal tubular cells are relatively impermeable to bicarbonate, so that filtered bicarbonate cannot be reabsorbed directly. Instead, carbonic acid is generated from carbon dioxide and water in renal tubular cells (catalysed by carbonate dehydratase) and dissociates into hydrogen and bicarbonate ions; the former cross the luminal cell membranes in exchange for actively reabsorbed sodium and react with filtered bicarbonate, generating carbon dioxide. This can diffuse into tubular cells and, together with the carbon dioxide produced by aerobic metabolism, provides the substrate for the continued formation of carbonic acid. Although the equilibrium for the reaction favours carbon dioxide and water, the continual removal of hydrogen ions drives the enzyme-catalysed reaction in the direction of hydrogen and bicarbonate ions. The bicarbonate thus formed accompanies sodium as it is pumped across the basolateral membranes of tubular cells into the interstitial fluid. The net effect is the reabsorption of filtered bicarbonate ions, with an equivalent amount of sodium ions. In health, bicarbonate ‘reabsorption’ takes place almost entirely in the proximal renal tubules. This process is summarized in Figure 5.2. When bicarbonate reabsorption is complete, continued production of hydrogen ions by renal tubular cells and their movement into the tubular fluid will constitute net hydrogen ion excretion. Acidification of the urine is achieved by active secretion of hydrogen ions and hydrogen ion/potassium ion exchange by the α-intercalated cells of the distal tubules and proximal parts of the collecting ducts. There is, however, a limit to the acidity of urine that can be achieved. This is a pH of approximately 4.5, equivalent to a hydrogen ion concentration of 38 μmol/L. This represents a 1000-fold concentration gradient with respect to the extracellular fluid, but clearly, excretion of free hydrogen ions alone would be insufficient to remove the daily burden of acid produced by metabolic processes, which is measured in millimoles. Significant acid excretion is achieved by hydrogen ions being buffered by phosphate, titrating monohydrogen phosphate Although the urine contains ammonium and, indeed, the amount excreted increases considerably in states of chronic acidosis, this cannot, for the reasons indicated above, in itself, constitute net hydrogen ion excretion. Ammonium is produced in renal tubular cells by the action of the enzyme glutaminase on glutamine, the amide of glutamic acid (Eqn. 27), and the oxidative deamination of glutamate by glutamate dehydrogenase (Eqn. 28). Glutamate is formed by transamination of 2–oxoglutarate with other amino acids, a process that does not involve either the production or utilization of hydrogen ions, and the equation for glutamine synthesis is the reverse of Equation 27. Thus, glutamine synthesis is a mechanism for the disposal of ammonium ions that does not (unlike urea synthesis) produce hydrogen ions. Although subsequent urinary ammonium excretion appears to be a means whereby hydrogen ions can be excreted in a buffered form, it does not represent direct excretion of hydrogen ions: rather, it is a process through which nitrogen can be excreted without the concomitant generation of hydrogen ions. As indicated in Equations 27 and 28, the production of ammonium from glutamine also yields 2–oxoglutarate: this is a substrate for gluconeogenesis, a process that consumes hydrogen ions. In acidosis, hepatic glutamine synthesis is increased: in the kidneys, the formation of ammonium from glutamine, urinary ammonium excretion and gluconeogenesis are all increased. The net result is a decrease in hydrogen ion formation and an increase in bicarbonate generation, both of which tend to correct the acidosis. Renal ammonium excretion is illustrated in Figure 5.4. The luminal membranes of renal tubular cells are actually impermeable to ammonium ions, but are permeable to ammonia. Continued diffusion of small amounts of ammonia in equilibrium with ammonium within renal tubular cells into the tubular fluid, results in continued formation of ammonia from ammonium. In the lumens of nephrons, this ammonia is immediately converted back to ammonium ions. This process does not entail net excretion of hydrogen ions; indeed, the net result is the same as if ammonium ions were transported directly. Traditionally, the kidneys have been considered (together with the lungs) as the major organs responsible for hydrogen ion homoeostasis, but the liver also plays a role, although its extent remains controversial. While the kidneys are the only organs capable of excreting hydrogen ions from the body, the liver both generates and consumes hydrogen ions. As indicated above, it has a central role in the production (e.g. ketogenesis, ureagenesis) and utilization of hydrogen ions (e.g. gluconeogenesis), yet an abnormal hydrogen ion concentration is an uncommon finding in patients with liver failure unless this is severe or there is accompanying renal disease. But this should not be surprising, since it is well known that the liver has substantial reserve capacity, and functional impairment may only occur with massive liver damage. There is certainly evidence that acid–base status has an influence on hepatic urea synthesis and glutamine synthesis, and affects lactate and ketone metabolism. For example, acidosis tends to stimulate hepatic glutamine synthesis and lactate disposal, but inhibits ketogenesis. Three organs are involved in hydrogen ion homoeostasis: the lungs, the kidneys and the liver. Extracellular hydrogen ion concentration depends on the ratio Nearly all the carbon dioxide produced each day is excreted through the lungs in the expired gas, although the liver is capable of disposing of a very small amount through the anaplerotic carboxylation of pyruvate to oxaloacetate. Although the liver can both generate and utilize hydrogen ions in metabolism, only the kidneys can excrete hydrogen ions from the body. In terms of the total turnover of hydrogen ions, the amount excreted this way is small. Nevertheless, it is vitally important, and a failure of renal hydrogen ion excretion frequently leads to acidosis, although even in acute kidney injury, if there are no additional causes of acidosis, it can take several days before the acidosis itself becomes severe. Whilst any one of abnormal respiratory, hepatic or renal function may lead to the development of acidosis or alkalosis, changes in the others may ameliorate the effect on arterial hydrogen ion concentration. The compensatory hyperventilation seen in patients with non-respiratory acidosis is an example of this. Such compensatory processes are of vital importance in disturbances of hydrogen ion homoeostasis, and are discussed in relation to the specific conditions in which they occur. However, it is important to appreciate that, although they may restore an abnormal hydrogen ion concentration to, or at least towards, normal, they do not correct the underlying disturbance. None of the clinical features of acidosis and alkalosis is specific to these disturbances and they may only be present when the disturbances are severe. The conditions that give rise to acidosis and alkalosis may have specific features, but, while clinical assessment is important in patients with disturbances of hydrogen ion homoeostasis, laboratory investigations are vital for their diagnosis, the assessment of their severity and for monitoring their progress. Measurements of arterial blood hydrogen ion concentration (strictly, activity) and PaCO2 (the ‘a’ indicates arterial, and ‘A’ alveolar; in this chapter, for the sake of simplicity, the ‘a’ is omitted except where doing so could lead to ambiguity) are fundamental to the assessment of acid–base status. Analysers may express hydrogen ion concentration as pH, but the SI unit is concentration and the use of concentration greatly facilitates data interpretation. Since the hydrogen ion concentration is determined by the ratio of PCO2 to bicarbonate concentration, bicarbonate concentration is not an independent variable and knowledge of it is not necessary for the characterization of acid–base disorders. Analysers calculate bicarbonate from PCO2, [H+] and Ka′, using the formula given in Equation 7. However, although Ka′ is supposedly a constant, there is evidence that it can vary unpredictably, particularly in severely ill patients. True bicarbonate concentration is not readily measurable. Most laboratory measurements of ‘bicarbonate’ are in fact measurements of total carbon dioxide (TCO2), to which bicarbonate makes by far the greatest contribution, but which also measures dissolved carbon dioxide, carbonic acid and carbamino compounds (histidine residues in proteins that have combined with carbon dioxide). Together, all these other species contribute only about 10% to the TCO2. It should be noted that the TCO2 concentration of plasma and serum decreases in vitro, as carbon dioxide is lost to the atmosphere. Caution should thus be exercised in interpreting its value if there has been any significant delay between the times of the blood sample being taken and the plasma or serum being analysed. Arterial blood for analysis must be suitably anticoagulated; any gas bubbles must be expelled and the specimen protected from the atmosphere and either analysed immediately using a point of care instrument, or transported rapidly to the laboratory, chilled by placing the syringe in which it is collected in iced water. Analysers that measure hydrogen ion concentration and PCO2 are generically termed ‘blood gas analysers’; they also measure PO2 and may provide other information of value in determining arterial oxygen content. They also frequently generate various derived terms, including ‘standard bicarbonate’, ‘base excess’ and ‘standard base excess’. These are calculated terms and add nothing to the characterization of disorders of hydrogen ion homoeostasis beyond what can be determined from consideration of [H+] and PCO2. As will be seen, bicarbonate can be affected by both respiratory and non-respiratory disturbances. The standard bicarbonate is the bicarbonate concentration that would be expected in that blood sample were the PCO2 to be normal. It supposedly eliminates any contribution by a respiratory disturbance, so that an abnormal standard bicarbonate concentration is taken as indicating the presence of a non-respiratory disturbance of hydrogen ion homoeostasis. The base excess is the calculated amount of acid in millimoles that would have to be added to 1 L of the patient’s blood in vitro to restore the hydrogen ion concentration to normal in an alkalosis (the base deficit is the corresponding figure for alkali in an acidosis). The standard base excess extends the base excess to include the whole extracellular compartment. The calculation of these derived variables makes unwarranted assumptions, and the data themselves can confuse, rather than facilitate, the analysis of clinical disorders. The only measurement required to derive these variables in addition to PCO2 and [H+] is the haemoglobin concentration. Since, therefore, derived units do not incorporate any other independent measurements of acid–base status, it follows that they cannot provide any information that cannot be derived from the measured variables alone. Furthermore, although it is correct that abnormal values for standard bicarbonate and base excess indicate the presence of a non-respiratory component in an acid–base disorder, they do not distinguish between this being a primary disorder or a compensatory response to a primary respiratory disorder. Base deficit is sometimes used to calculate the amount of bicarbonate that is required to correct an acidosis, but, in practice, if an acidosis is so severe that bicarbonate treatment is warranted, this should be given in small amounts and the effect assessed by frequent measurements of [H+] and PCO2. The anion gap is the sum of the concentrations (in mmol/L) of the two major cations in plasma minus those of the two major anions (i.e. Determination of the anion gap can be of help in determining the cause of a non-respiratory acidosis. When acidosis is due to loss of bicarbonate (e.g. renal tubular acidoses, loss of bicarbonate from the gut), there is increased renal chloride retention; the lost bicarbonate is replaced by chloride and the anion gap is unaffected. On the other hand, when acidosis is due to ingestion or excess generation of acids, the associated anions (e.g. lactate) replace bicarbonate as this is consumed by buffering, and the anion gap is increased. In practice, determination of the anion gap is only likely to be helpful if the cause of the acidosis is not already obvious. It certainly does not substitute for careful clinical assessment. For its calculation, plasma chloride concentration must be known, and in the UK, this often must be requested individually, rather than being available as part of a profile of tests. Also, the imprecision of the value of the anion gap, summating as it does the individual imprecisions of the measurements of the analytes that are required to calculate it, needs to be taken into account. Other biochemical measurements may be of value in the assessment of disorders of hydrogen ion homoeostasis under certain conditions, for example in patients with diabetes, in neonates and in poisoned patients. These measurements are discussed in the sections that follow. Although they differ in their pathogenesis, an understanding of the disorders of hydrogen ion homoeostasis is facilitated by considering them in a similar way. The processes involved are the generation of the disorder, buffering, physiological compensation and ultimate correction. In practice, there is often overlap between these. Disorders of hydrogen ion homoeostasis may be simple, that is, involving only one type of disturbance, or mixed (two or more disturbances arising together). Unless rapidly corrected, simple disturbances typically generate secondary changes, which, in terms of bicarbonate concentrations and PCO2, can take on the characteristics of mixed disturbances. This disorder can develop as a consequence of any, or a combination of, an increase in the rate of generation of hydrogen ions, a decrease in the rate of their utilization or excretion or a decrease in buffering capacity. A list of some of the causes of non-respiratory acidosis is given in Box 5.1. Frequently, more than one cause may contribute to an acidosis in an individual patient. For example, patients with diabetic ketoacidosis are usually hypovolaemic and their renal function is impaired. Many of these conditions are discussed in detail in other chapters of this book; the reasons why they can cause acidosis should be evident from the first section of this chapter. Some conditions of particular interest are discussed later. Although many of the conditions causing acidosis have distinct clinical features, some of the features of acidosis, and of the body’s response to it, are common to them all. Non-respiratory acidosis can develop rapidly, particularly when it is due to increased production of hydrogen ions or loss of bicarbonate. A tendency for hydrogen ion concentration to increase will be resisted by buffering; bicarbonate will be consumed and its concentration in the plasma will tend to fall. In the early stages of a condition that can cause acidosis, buffering, together with increased renal hydrogen ion excretion and bicarbonate regeneration, may prevent the development of a significant rise in hydrogen ion concentration. In chronic acidosis, buffering of hydrogen ions by tissue proteins also plays an important part in limiting the rise in hydrogen ion concentration. Provided that respiratory function and its control are normal, acidosis stimulates ventilation through effects on aortic and carotid body chemoreceptors, and directly on the respiratory centre. Kussmaul breathing – deep, sighing respiration – is characteristic of non-respiratory acidosis. This not only allows excretion of carbon dioxide derived from the carbonic acid produced by buffering, but actually reduces the PCO2, which in turn tends to lower the hydrogen ion concentration towards normal. The muscular effort of hyperventilation itself generates carbon dioxide, and there is a lower limit to the PCO2
Hydrogen ion homoeostasis and tissue oxygenation and their disorders
INTRODUCTION
THE PHYSIOLOGICAL ROLE OF HYDROGEN IONS
Definitions
HYDROGEN ION HOMOEOSTASIS
Buffering
Bicarbonate
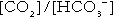 is approximately 0.05, suggesting that this system should be a poor buffer, particularly against a tendency for hydrogen ion concentration to fall. However, the body is a net producer of acid, that is, the tendency is for hydrogen ion concentration to rise. More importantly, carbon dioxide, generated by the buffering of hydrogen ion by bicarbonate and the subsequent dissociation of carbonic acid, can be removed by the lungs, keeping the carbon dioxide concentration constant. As a result, the effective buffering capacity of the carbonic acid–bicarbonate system is greatly increased, and it is a vitally important physiological buffer, particularly in the extracellular fluid.
is approximately 0.05, suggesting that this system should be a poor buffer, particularly against a tendency for hydrogen ion concentration to fall. However, the body is a net producer of acid, that is, the tendency is for hydrogen ion concentration to rise. More importantly, carbon dioxide, generated by the buffering of hydrogen ion by bicarbonate and the subsequent dissociation of carbonic acid, can be removed by the lungs, keeping the carbon dioxide concentration constant. As a result, the effective buffering capacity of the carbonic acid–bicarbonate system is greatly increased, and it is a vitally important physiological buffer, particularly in the extracellular fluid.
Phosphate
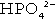 and
and 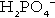 ) form a buffer pair with Ka being approximately 160 nmol/L. Although the Ka appears relatively favourable for buffering at physiological hydrogen ion concentrations, the concentration of phosphate in the extracellular fluid is too low for it to be of significance in this respect. Phosphate is, however, an important buffer in the urine, where its concentration is much greater.
) form a buffer pair with Ka being approximately 160 nmol/L. Although the Ka appears relatively favourable for buffering at physiological hydrogen ion concentrations, the concentration of phosphate in the extracellular fluid is too low for it to be of significance in this respect. Phosphate is, however, an important buffer in the urine, where its concentration is much greater.
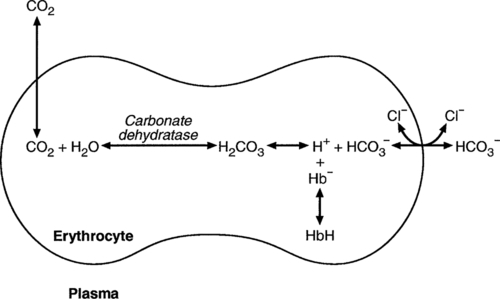
Haemoglobin
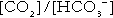 (and hence in hydrogen ion concentration) between arterial and venous blood. However, although erythrocytes are a potential source of bicarbonate, they can only generate bicarbonate if the hydrogen ion produced simultaneously can be buffered. There is clearly a limit to the extent to which this can occur, imposed by the buffering capacity of haemoglobin, so that this mechanism can have only a limited role in the correction of an acidosis. The way in which such correction is achieved is considered in a later section.
(and hence in hydrogen ion concentration) between arterial and venous blood. However, although erythrocytes are a potential source of bicarbonate, they can only generate bicarbonate if the hydrogen ion produced simultaneously can be buffered. There is clearly a limit to the extent to which this can occur, imposed by the buffering capacity of haemoglobin, so that this mechanism can have only a limited role in the correction of an acidosis. The way in which such correction is achieved is considered in a later section.
Other proteins
Ammonia
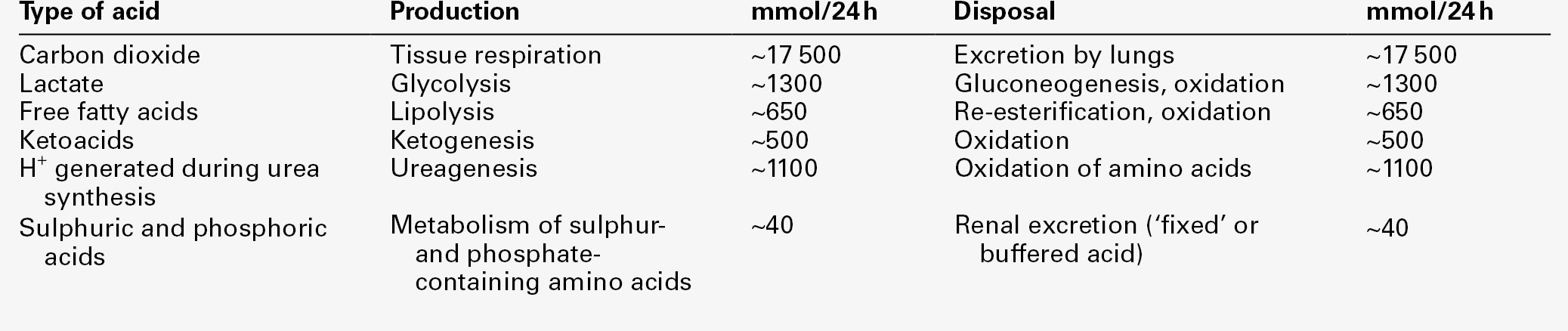
Hydrogen ion turnover
Hydrogen ion production
Carbon dioxide
Incomplete metabolism of glucose: glycolysis and lactate metabolism
Incomplete metabolism of triglycerides: ketogenesis
Complete oxidation of glucose and triglycerides
Amino acid metabolism
Hydrogen ion excretion
Carbon dioxide
Hydrogen ions
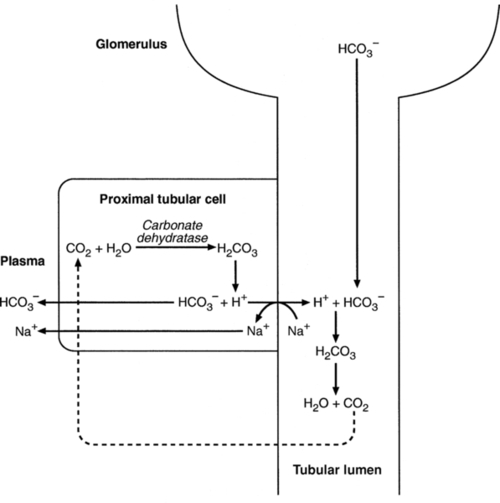
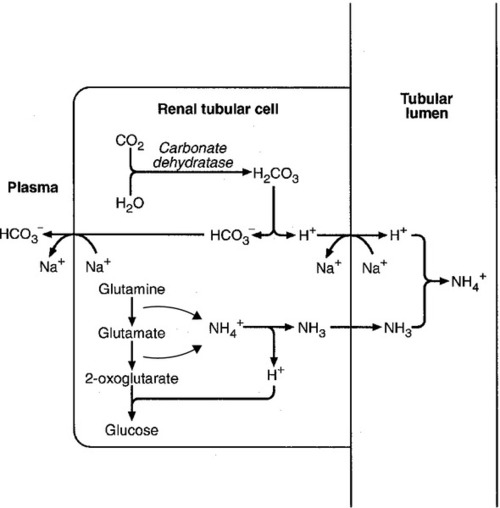
Bicarbonate reabsorption
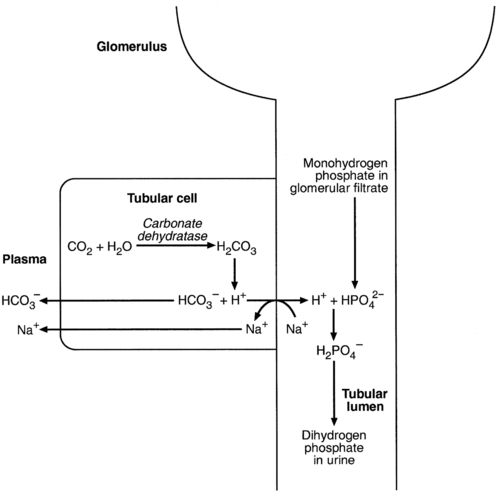
Acidification of the urine
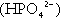 (the principal form in the plasma and thus the glomerular filtrate) to dihydrogen phosphate
(the principal form in the plasma and thus the glomerular filtrate) to dihydrogen phosphate 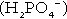 ions. It should be noted that, since the formation of hydrogen ions in renal tubular cells is accompanied by stoichiometric generation of bicarbonate ions, the excretion of hydrogen ions additionally results in the regeneration of bicarbonate ions and thus restores buffering capacity. This process is summarized in Figure 5.3.
ions. It should be noted that, since the formation of hydrogen ions in renal tubular cells is accompanied by stoichiometric generation of bicarbonate ions, the excretion of hydrogen ions additionally results in the regeneration of bicarbonate ions and thus restores buffering capacity. This process is summarized in Figure 5.3.
The role of urinary ammonium excretion
The role of the liver in hydrogen ion homoeostasis
Summary
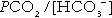 . The respiratory centre is exquisitely sensitive to arterial PCO2 and, given normal respiratory function, PCO2 is maintained within narrow limits by changes in the rate and depth of respiration. It has been calculated that cessation of carbon dioxide excretion would lead to potentially fatal acidosis within 30 min (although this is purely hypothetical, since the accompanying failure in oxygen supply would be fatal before this time).
. The respiratory centre is exquisitely sensitive to arterial PCO2 and, given normal respiratory function, PCO2 is maintained within narrow limits by changes in the rate and depth of respiration. It has been calculated that cessation of carbon dioxide excretion would lead to potentially fatal acidosis within 30 min (although this is purely hypothetical, since the accompanying failure in oxygen supply would be fatal before this time).
THE ASSESSMENT OF ACID–BASE STATUS
Clinical assessment
Laboratory assessment
Hydrogen ion concentration and PCO2
Derived variables
Anion gap
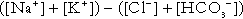 ). The total concentration of anionic charge in plasma must equal the concentration of cationic charge: the anion gap reflects mainly the anionic nature of most proteins in plasma at physiological hydrogen ion concentration, although phosphate and other anions make a small contribution. Its normal value is related to the concentrations of the species that determine it, and is ~ 15 mmol/L.
). The total concentration of anionic charge in plasma must equal the concentration of cationic charge: the anion gap reflects mainly the anionic nature of most proteins in plasma at physiological hydrogen ion concentration, although phosphate and other anions make a small contribution. Its normal value is related to the concentrations of the species that determine it, and is ~ 15 mmol/L.
Other investigations
DISORDERS OF HYDROGEN ION HOMOEOSTASIS
Introduction
Non-respiratory acidosis
Compensatory responses in non-respiratory acidosis
Buffering
Hyperventilation
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree