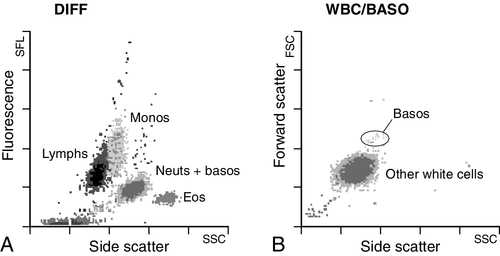
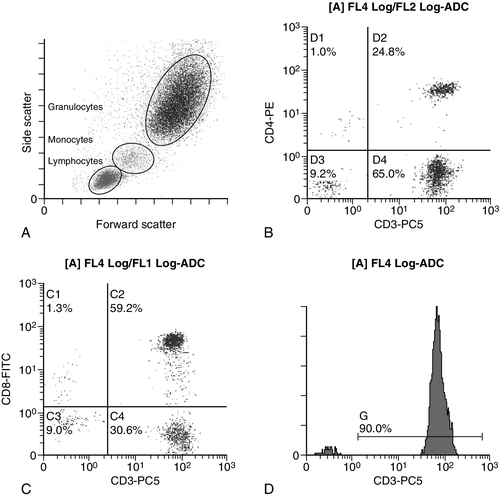
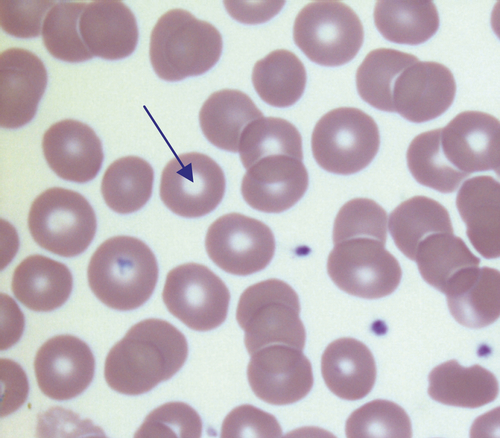
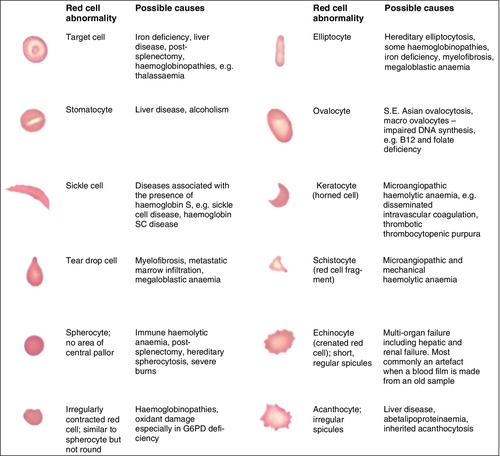
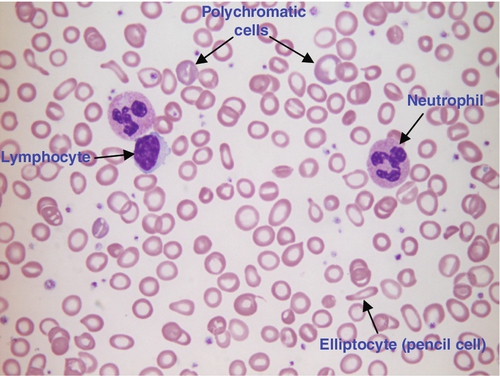
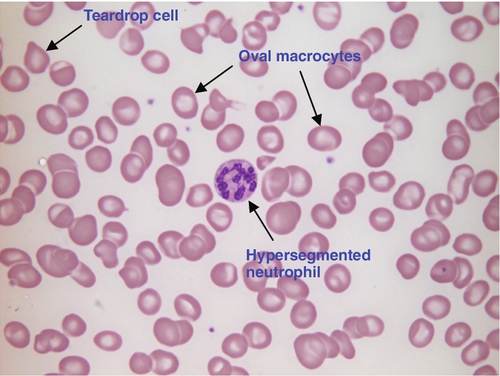
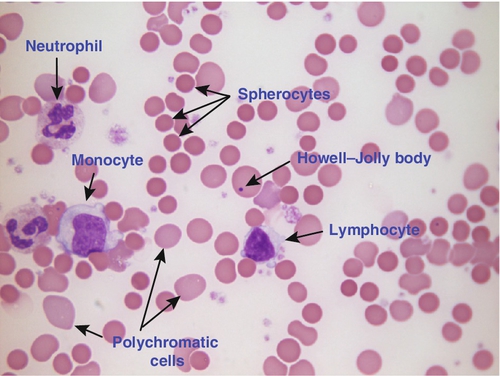
CHAPTER 26 David Ah-Moye; Ceinwen Davies; Joanne Goody; Peter Hayward; Rebecca Frewin CHAPTER OUTLINE Analysis of the full blood count Erythrocyte sedimentation rate and plasma viscosity Tests for infectious mononucleosis Abnormal white cell morphology Laboratory tests of coagulation Interpretation of coagulation tests Investigation of suspected transfusion reaction Haemolytic disease of the newborn Haematology is the study of blood, its formation, composition, functions and diseases. Blood cell formation (haemopoiesis) is described in Chapter 27. Haematology laboratories perform a wide range of tests of the composition and function of the blood that assist the clinician in the diagnosis and management of disease. They are typically divided into three principal sections: general haematology, haemostasis and blood transfusion. This chapter provides an overview of the different diagnostic techniques offered in each. The general haematology laboratory performs numerical, morphological and biochemical analysis of blood samples (now often as part of a combined blood sciences laboratory). Blood samples must be anticoagulated, for example with ethylenediamine tetra-acetic acid (EDTA) for determination of full blood count and reticulocyte count, blood film examination and plasma viscosity and tri-sodium citrate for coagulation tests and measurement of erythrocyte sedimentation rate. The full blood count (FBC) is the most requested test profile in haematology. It comprises: • haemoglobin (Hb) concentration • red blood cell (RBC) count • red cell indices (volume and haemoglobin content) • white blood cell (WBC) count • white blood cell differential count (neutrophils, lymphocytes, monocytes, eosinophils and basophils) • platelet count. The FBC is a valuable first-line screening test from which further investigations may be generated (see Table 26.1). The clinician uses information from the FBC in conjunction with the clinical history, physical examination and other investigations to help formulate and distinguish between differential diagnoses. Full blood counts are usually performed on fully automated analysers, which provide accurate quantitation of cell parameters and can generate alerts, commonly known as ‘flags’, to the presence of unusual characteristics that may warrant review of a blood film. The main function of haemoglobin, which is present solely within the red cells, is to transport oxygen from the lungs to the tissues (see Chapter 5). Haemoglobin is measured by spectrometry. Diluted whole blood is mixed with a reagent (e.g. Stromatolyser™) that results in cell lysis and conjugates the haemoglobin with a compound such as potassium cyanide or sodium lauryl sulphate prior to measurement of its absorbance. The presence of high concentrations of substances that increase absorbance, e.g. triglyceride-rich lipoproteins, may cause a falsely raised haemoglobin result with some methods. Blood cells are counted and sized either by electrical impedance or laser light scattering methods. The impedance method utilizes the fact that blood cells are very poor conductors of electricity. When a cell passes through a small aperture across which an electric current is applied, there is a measurable rise in the electrical impedance, with the pulse height being proportional to the volume of the cell. Each cell generates a separate impulse. Light scatter utilizes the fact that cells scatter light when passed through a laser beam: the number of cells can be counted and information about the cells can be obtained according to the pattern of scatter. White cell counting is performed after lysis of the red cells. Clumping of cells results in underestimation of cell numbers. This may occur, for example, as a result of autoagglutination of red cells in cold agglutinin disease or in vitro clumping of platelets because of inadequate mixing with EDTA anticoagulant. The red cell indices are a combination of measured and derived red cell parameters. The red cell indices usually reported are: • haematocrit (HCT) or packed cell volume (PCV), the percentage volume of red cells in the blood • mean cell haemoglobin (MCH), the average mass of haemoglobin per red cell • mean cell haemoglobin concentration (MCHC), the average concentration of haemoglobin per red cell. Some analysers measure the MCV and calculate the HCT or PCV; others measure the HCT and calculate the MCV. is useful when assessing anaemia. For example, it is reduced in iron deficiency anaemia and increased in megaloblastic anaemia. is reduced in iron deficiency anaemia and thalassaemias; further tests such as ferritin and HbA2 measurement may be performed to differentiate between these two conditions. is reduced in iron deficiency but is probably a more useful tool within the laboratory than clinically. It is very stable in health, so it can be used as an internal quality control; a significant change in the daily mean MCHC indicates a drift in analyser calibration or a fault in its function. The MCHC is also useful for detecting anomalous results: a falsely raised Hb concentration, for example as a consequence of lipaemia, or a falsely reduced RBC count, for example owing to red cell autoagglutination, will result in a falsely raised MCHC. The formulae for the derived parameters are as follows: The white blood cells are involved in the cellular immunological response to infection and other causes of inflammation. The five types of white blood cell differ in volume, conductivity, light scattering properties, uptake of fluorescent dyes and cytochemical stains, and resistance to lytic agents. These properties, used in various combinations, enable their identification. Figure 26.1 shows typical white cell scattergrams for a normal blood sample. FIGURE 26.1 Five-part WBC differential from Sysmex XE2100TM analyser. Note that neutrophils and basophils cannot be separated in the side fluorescence (SFL) vs side scatter (SSC) population scattergram (A). The basophil count is obtained by treating the white cells with an acidic reagent that shrinks all white cells, leaving bare nuclei, except for basophils, which remain intact (B). The neutrophil count is then derived by subtracting the basophil count from the combined neutrophil and basophil count. Lymphs, lymphocytes; monos, monocytes; neuts, neutrophils; eos, eosinophils; basos, basophils. The main function of platelets is to form a plug at sites of damage to vascular endothelium. A low platelet count (thrombocytopenia) may result from decreased production, increased consumption or abnormal pooling in the spleen. Decreased production is a consequence of either general marrow failure or selective depression, for example by viral infection, drugs and chemicals. Platelet consumption is a feature of certain types of autoimmune diseases and occurs in disseminated intravascular coagulation (which is often a result of septicaemia) and thrombotic thrombocytopenic purpura. A high platelet count (thrombocytosis) may be seen as part of a reactive process such as infection or in myeloproliferative neoplasms. Automated haematology analysers routinely measure the platelet count by impedance or laser light scattering; the reference method uses flow cytometry after labelling the platelets with CD41 or CD61 antibodies. Reticulocytes are immature red cells, which are released from the bone marrow into the circulation one to two days before maturation. An increase in the reticulocyte count is indicative of an increase in red cell production (erythropoiesis) to meet physiological demand, for example in haemolytic anaemia. Reticulocytes contain ribosomal RNA, which can be detected by two main methods. When unfixed red cells are incubated with dyes such as new methylene blue, the ribosomal RNA precipitates out and appears as a blue reticular network within the cells, which can be visualised using a light microscope, allowing the reticulocytes to be counted. Most automated FBC analysers now perform reticulocyte counts by flow cytometry, using a fluorescent dye that binds to the ribosomal RNA; the number of reticulocytes can be expressed as a percentage of total red cells and as an absolute count. expressed in mm/h, is a measure of the rate at which red blood cells settle when an anticoagulated blood sample is left to stand in a column. is usually measured by an automated viscometer. The plasma is drawn through a capillary tube under constant pressure and temperature; the rate of flow is measured and expressed as viscosity of plasma relative to distilled water. Measurement of ESR or plasma viscosity may be useful when assessing the acute phase response. Both are affected by the presence of large proteins such as fibrinogen, α2-macroglobulin and immunoglobulins, and are therefore usually raised during an acute phase response. They are non-specific screening tools for inflammation, and are useful in monitoring of diseases such as rheumatoid arthritis and response to treatment for example with non-steroidal anti-inflammatory drugs and anti-tumour necrosis factor. Plasma viscosity has several advantages over ESR. For example, it is not affected by physiological and environmental factors. Measurement is rapid, reproducible and lends itself better to quality assurance procedures than measurement of ESR. Conversely, ESR is a cheap test, but is influenced by factors such as anaemia, sex, age and (in females) stage of the menstrual cycle and must be carried out within about six hours of venepuncture. White cells express characteristic nuclear, cytoplasmic and surface antigens; this is referred to as the immunophenotype of the cell. Immunophenotyping has been made possible by the availability of a large number of antibodies, which target specific cellular antigens. For example, antibodies that are directed against the CD19 antigen will bind to B lymphocytes. Immunofluorescence forms the basis of flow cytometric investigations. It uses antibodies conjugated to fluorescent dyes (fluorochromes) such as fluorescein isothiocyanate (FITC), phycoerythrin (PE) and phycoerythrin cyanin (PC). Whole blood or bone marrow is incubated with one or more antibodies, each conjugated to a different fluorochrome. The cells are then injected in single file into the path of a laser beam. As the cell passes through, the light is scattered in all directions. Forward scatter (FSC) is influenced by cell size whereas side scatter (SSC) is influenced by the cellular structure and contents. The different cell populations can be visualized by plotting SSC against FSC. As the cell–antibody complex passes through the laser beam it fluoresces. The different wavelengths of light emitted are filtered, enabling specific cell populations to be selected (gated) and the data can be displayed as dot plots or histograms. The dot plots show the intensity of fluorescence as well as the number of cells that are positive for the particular antigen; the intensity of fluorescence is proportional to the strength of antigen expression. In the example shown in Figure 26.2, whole blood was incubated with three antibody-dye conjugates, CD8-FITC, CD4-PE and CD3-PC5. FIGURE 26.2 Scatter plots, dot plots and histogram printouts from Beckman Coulter FC500 flow cytometer. (A) Side scatter vs forward scatter, showing white cell populations. (B) CD4 vs CD3 dual dot plots. (C) CD8 vs CD3 dual dot plots. (D) Single histogram for CD3 gated for the lymphocyte population. Note: Granulocytes is the collective name for neutrophils, eosinophils and basophils. Flow cytometry allows rapid immunophenotyping of cells. This is essential in the diagnosis and classification of leukaemias and lymphomas, but is also a useful tool in the assessment of non-malignant conditions and for monitoring the CD4 + lymphocyte count during the treatment of human immunodeficiency virus (HIV) infection. Flow cytometry can also be used to confirm feto–maternal haemorrhage (see p. 512), by the detection of Rh D positive fetal cells and measurement of haemoglobin F in the fetal cells. These comprise direct measurement of the concentration of ferritin (a measure of iron status), vitamin B12 and folate. They are discussed further in Chapter 27. The haemoglobinopathies and the techniques used for their diagnosis are discussed in Chapter 29. Infectious mononucleosis (IM), also known as glandular fever, is caused by the Epstein–Barr virus (EBV), which infects B lymphocytes. It is associated with a high titre of a heterophile antibody, so-called because it also reacts with red cells of other species, for example sheep, ox and horse. This antibody has been termed the Paul–Bunnell antibody, named after John Paul and Walls Bunnell who discovered them. The Paul–Bunnell antibody is not adsorbed by guinea pig kidney, in contrast to other heterophile antibodies (Forssman antibodies). Differential adsorption tests utilize this principle; the patient’s serum is incubated with ox red cells and separately with guinea pig kidney antigen, after which horse red cells (reagent) are added to the mixtures. Red cell agglutination only in the latter mixture indicates the presence of the Paul–Bunnell antibody. An alternative test is the latex agglutination test. A reagent containing latex microspheres coated with purified Paul–Bunnell antigen is mixed with the patient’s serum; agglutination indicates presence of the Paul–Bunnell antibody. Definitive confirmation of EBV infection requires serological detection of a rise in the titre of specific anti-EBV capsid antigen IgM, which occurs in the first few weeks following infection. Microscopic examination of a well stained blood film is an essential part of many haematological investigations. The film may be made either manually or by an automated slide maker. After being air dried, it is stained with Romanowsky dyes such as May–Grünwald Giemsa or modified Wright stain, which contain azure B and eosin. This enables the assessment of the size, shape, maturity and contents of blood cells by light microscopy. Blood film examination is useful in the investigation of the causes of anaemia and to diagnose white cell disorders such as leukaemia and parasitic infections such as malaria. Less commonly, a blood film may be requested for specific reasons such as to look for acanthocytes (red cells with irregular spicules), which are associated with abetalipoproteinaemia, liver disease and several rare degenerative neurological diseases. In health, there is little variation in the shape and size of red blood cells. On a well spread and stained blood film, red cells appear circular in outline, with a well stained outer rim and a paler central area that occupies approximately a third of the cell (see Fig. 26.3). The normal red cell appears slightly smaller than the nucleus of a small lymphocyte. It is referred to as normocytic (normal size) and normochromic (normal staining characteristics). FIGURE 26.3 Normal red cells. Shows area of central pallor (arrow) occupying one-third the size of the cell. Valuable information can be obtained from the shape of a red cell, as shown in Figure 26.4. In many haematological conditions there may be an increase in the proportion of non-specifically abnormal red cells, termed poikilocytosis. It is also good practice to check the patient’s biochemistry results, to corroborate the morphological features; for example, if acanthocytes are present, the liver function tests should be checked, as acanthocytes are commonly present in liver disease. FIGURE 26.4 Red blood cells. Commonly observed morphological changes in red cells and the disorders in which they arise. Anaemia is a condition in which the number of red blood cells, or their oxygen-carrying capacity, is insufficient to meet physiological needs. This is conventionally defined as a reduction in haemoglobin concentration below the reference range, i.e. < 130 g/L in adult males and < 115 g/L in adult females. The causes and assessment of anaemia are discussed in Chapter 27. In iron deficiency anaemia, the red cells are smaller than normal (microcytosis). This is because the maturing red cells undergo an extra cellular division before the critical haemoglobin concentration required to arrest mitosis is achieved. The cells are also hypochromic, with a larger area of central pallor (see Fig. 26.5). Polychromatic cells (immature red cells that have a bluish hue on routine staining because of retained ribosomal ribonucleic acid) may be present, indicating increased erythropoiesis. FIGURE 26.5 Iron deficiency anaemia (IDA). Hb 58 g/L, MCV 52.1 fL, MCH 12.7 pg, PLT 608 × 109/L, ferritin < 5 μg/L. Blood film shows microcytic hypochromic red cells, polychromatic red cells and pencil cells. The film also shows two neutrophils, a lymphocyte and an increase in platelet numbers. Megaloblastic anaemia is commonly caused by deficiency of vitamin B12 or folate, both of which are essential for DNA synthesis, or the administration of drugs that interfere with DNA synthesis (e.g. methotrexate). Defective DNA synthesis results in the nucleus maturing at a slower rate than the cytoplasm, thereby producing a red cell that is larger than normal (macrocyte). Teardrop cells and red cell fragments may also be seen, as a consequence of ineffective erythropoiesis (see Fig. 26.6). A common finding is the presence of hypersegmented neutrophils (defined as presence of a nucleus with more than five lobes). Haemolytic anaemia is a term used to describe anaemia caused by a shortened red cell lifespan. In autoimmune haemolytic anaemia, the red cells are coated with antibody (usually IgG) that is directed against the patient’s own cells. When these cells circulate through the reticuloendothelial system, the surface of the red cells is eroded, leading to a change in their shape from a biconcave disc to a sphere (see Fig. 26.7). FIGURE 26.7 Warm autoimmune haemolytic anaemia. Hb 104 g/L, reticulocytes 604 × 109/L, haptoglobin < 0.2 g/L (reference range 0.7–3.2), direct antiglobulin test positive. Blood film shows numerous spherocytes, polychromatic cells and a red cell containing a Howell–Jolly body. Howell–Jolly bodies are typically found in patients who have undergone splenectomy. The film also shows two neutrophils, a monocyte and a lymphocyte. Microangiopathic haemolytic anaemia is a term that is used to describe the anaemia that results from physical damage to the red cells following the occlusion of arterioles and capillaries as a result of fibrin deposition or platelet aggregation. There are numerous causes, including infections (resulting, for example in disseminated intravascular coagulation (Fig. 26.8
Introduction to haematology and transfusion science
INTRODUCTION
GENERAL HAEMATOLOGY
Analysis of the full blood count
Haemoglobin
Cell counting
Red cell indices
Mean cell volume
Mean cell haemoglobin
Mean cell haemoglobin concentration
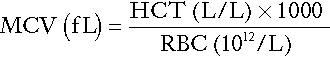
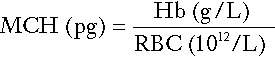
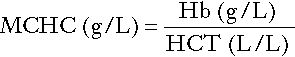
White cell differential
Platelet count
Reticulocyte count
Erythrocyte sedimentation rate and plasma viscosity
Erythrocyte sedimentation rate (ESR)
Plasma viscosity
Flow cytometry
Haematinic studies
Haemoglobinopathy screening
Tests for infectious mononucleosis
MORPHOLOGY
Blood film examination
Normal red cell morphology
Morphology of the anaemias
Iron deficiency anaemia
Megaloblastic anaemia
Autoimmune haemolytic anaemia
Microangiopathic haemolytic anaemia
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree