OUTLINE
Pharmaceutical Uses of Powders
Advantages of Solid Dosage Forms
Principles of Compounding for Powders
Compounding Divided Powders or Chartulae
Compatibility, Stability, and Beyond-Use Dating
I. DEFINITIONS
A. Powders: Powders are defined in USP 31 Chapter 〈1151〉, Pharmaceutical Dosage Forms, as “intimate mixtures of dry, finely divided drugs and/or chemicals that may be intended for internal (Oral Powders) or external (Topical Powders) use” (1). A similar definition is given in the CDER Data Standards Manual published by the FDA Center for Drug Evaluation and Research (CDER), http://www.fda.cder/dsm/DRG/drg00201.htm, accessed March 2008.
B. Powder: Proposed new USP nomenclature for pharmaceutical dosage forms defines the more general term powder as “a solid, or mixture of solids, that has been reduced to a finely divided state” (2). (See the introduction to Chapter 27 for an explanation and discussion of proposed revised USP nomenclature.)
C. The following powder types have physical properties that require special handling. In addition to information given in this chapter, compatibility and stability issues for these powders are discussed in Chapter 37.
1. Efflorescent powders are drugs or chemicals that contain water of hydration that may be released when the powders are manipulated or are stored under conditions of low relative humidity.
2. Hygroscopic powders are solid drugs or chemicals that absorb moisture from the air.
3. Deliquescent powders are hygroscopic powders that may absorb sufficient moisture from the air to dissolve and form a solution.
4. Pharmaceutical eutectic mixture is a mixture of two or more solid substances that may liquefy when intimately mixed at room temperature.
II. PHARMACEUTICAL USES OF POWDERS
A. Topical bulk powders are applied to the skin for local effect.
B. Bulk powders for internal use offer a convenient method of dispensing nonpotent, powdered drugs that have doses that require moderate to large volumes of powder. Common therapeutic uses for oral bulk powders include antacids, bulk laxatives, antidiarrheal medications, and oral electrolyte mixtures for rehydration. Bulk powders also offer a convenient way for patients to make vaginal douches. Because, at the time of administration, the patient or caregiver measures the dose volumetrically using a household measuring spoon or cup, this dosage form cannot be used for drugs that require precise and accurate dosing.
C. Powders may also be dispensed as divided powders, also known as chartulae. In this case, the compounder weighs each dose of powder separately and places it in a small individual packet. At the time of administration, the patient or caregiver mixes the contents of a packet into a liquid or other vehicle. When the medication is for oral use, the powder can be mixed with soft food, such as pudding or applesauce, for ease of administration. This dosage form is useful when a solid dosage form is desired but the medication is not manufactured in the required dose, or when it is supplied as capsules or tablets but the patient cannot swallow these dosage forms.
D. Powders for internal use may also be encapsulated into hard-shell capsules or compressed into tablets. Because the quantity of drug formulated into tablets and capsules can be measured accurately and precisely, these systems are ideal for potent drugs. Compounding of capsules is discussed in Chapter 26.
E. Because nearly all drugs are solids, powders are used as the primary ingredients for most other dosage forms.
III. ADVANTAGES OF SOLID DOSAGE FORMS
A. Drugs and chemicals are most stable as dry solids.
B. Because they are dry and compact, tablets, capsules, and divided powders are packaged, transported, administered, and stored more easily than are drugs formulated in solutions or suspensions.
C. Undesirable taste is less noticeable when substances are in solid form than when in solution. Objectionable taste can be concealed completely by enclosing the solid drug in capsules or coated tablets.
D. Accurate dosing is facilitated with dosage forms furnished as individual units, such as tablets, capsules, and divided powders.
E. Controlled release is much easier to achieve with solid dosage forms than with liquids.
IV. DESIRED PROPERTIES OF POWDERS
A. When intended for topical application, powders should be finely divided and have uniform particle size so as to be smooth to the touch and nonirritating to the skin. They should be free-flowing and should spread easily on the surface of the skin.
B. Powders for internal use should also be finely divided with uniform particle size because the rate of dissolution, and therefore often the bioavailability of the drug, depends on the particle size of the drug.
1. Dissolution rate is expressed mathematically by the Noyes-Whitney equation:
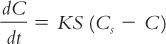
where

2. Because the surface area (S) of a given amount of solid increases as the particle size is decreased for a given weight of solid, the smaller the particle size, the larger the surface area and the faster the rate of dissolution.
C. Even when powder is being used as an ingredient for another dosage form, particle size is important. Not only does it affect the rate of dissolution and bioavailability in these preparations, but it also can affect the rate of settling (in suspensions) and the degree of comfort (in topical suspensions, ointments, and creams).
D. For bulk powders, it is important for the particle size to be uniform, because particles of different sizes tend to stratify on standing or when a powder is being transported. Stratification can result in inaccurate dosing.

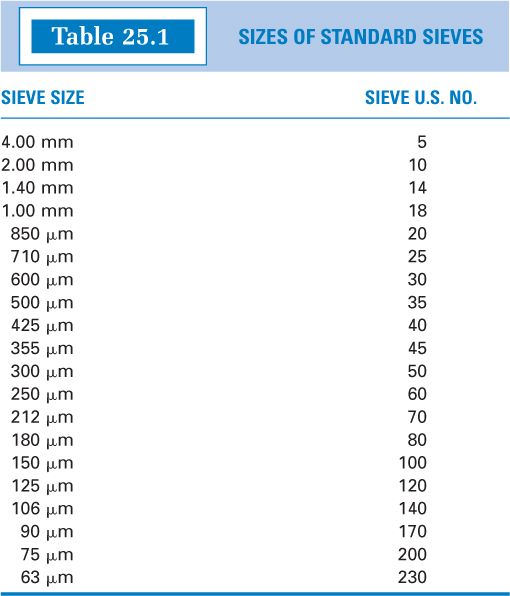
MESH SIEVES
E. Particle size for most pharmaceutical powders is determined by sieving, and the descriptive terms used to classify powders have meaning in terms of percent of the powder sample that will pass through a sieve of a given fineness.
1. Sieve properties are specified in the International Organization for Standardization Specifications ISO 3310-1:Test Sieves—Technical Requirements and Testing, and particle size determination using sieves is described in USP Chapter 〈786〉, Particle Size Distribution Estimation by Analytical Sieving. Table 25.1 shows the sieve size openings for sieves used to measured particle size for powders of interest in pharmaceuticals. The table column “Sieve U.S. No.” gives what is commonly referred to as mesh size, a number that comes from the number of openings per linear inch of the sieve mesh. The larger the mesh number, the smaller the sieve openings and the smaller the particles must be to pass through that sieve.
2. Powders almost never have a completely uniform particle size, but rather they have a size distribution. Therefore, even when using sieves to measure particle size, the results are reported as the percent of the sample that passes through a given sieve size plus an upper and lower size boundary, or specifications will state that all the powder must pass through a sieve of a given mesh size. For example, the USP compounding monograph for Ketoconazole Oral Suspension states, “If Tablets are used, finely powder the Tablets such that they pass through a 40-mesh or 45-mesh sieve” (3). USP also recommends that topical powders, at a minimum, should pass through a size 100-mesh sieve (1).
3. Descriptive terms, such as fine or coarse, are used to describe powder fineness. The classification used by the USP for this purpose is given in Table 25.2, where the designation d50 means “the smallest sieve opening through which 50% or more of the material passes” (4).
4. Small (3-inch-diameter) and medium (8-inch-diameter) sieves with mesh sizes useful for compounding are available at a modest price from some vendors of compounding supplies.
V. PRINCIPLES OF COMPOUNDING FOR POWDERS
A. General principles
1. In nearly all compounding situations, solids need to be in a fine state of subdivision. Unless the solid can be purchased as a fine powder, particle size reduction by the compounder is required.
2. The chemical composition and the processing of solids determine their degree of subdivision and physical properties. The properties of a given solid must be understood and considered to properly handle and manipulate the material when fabricating it into a solid dosage form or when incorporating it into another drug delivery system.
a. Solids that are purchased as fine powders may need no further manipulation.
b. Some drugs have fine particles, but these may have agglomerated on storage and may need to be broken down into the primary particles originally processed by the manufacturer.
c. Some drugs and chemicals are available as crystals that can easily be crushed into fine powder using a standard compounding technique, such as trituration, described in B. 2. a.
d. Some materials are not easy to pulverize. They may be waxy substances or hard crystals that do not crush into fine powder with simple trituration. If a fine state of subdivision is needed, these drugs may require special techniques, such as pulverization by intervention (see B. 2. b.).
e. Some solids have unique properties, such as deliquescence or intense color, that require special handling.
f. Some solid drugs and chemicals are cytotoxic or hazardous substances; these, too, require special precautions and handling.
3. When two or more solids are being combined into one mixture, homogeneous blending of the powders is needed.
B. Particle size reduction
1. The process of particle size reduction is called comminution. The pharmaceutical industry has elaborate equipment and processes with which to produce finely divided powders with precisely controlled particle size. Although the equipment and methods available in the pharmacy are not nearly as efficient, they work well for the processing done by pharmacists and pharmacy technicians in extemporaneous compounding.
2. Methods of comminution available in the pharmacy

a. Trituration is the continued rubbing of a solid in a mortar with a pestle to reduce the size of the solid’s particles to a desirable degree of fineness. The term is also used to describe the grinding together of two or more substances in a mortar to intimately mix them. Trituration is achieved by firmly holding the pestle and exerting a downward pressure with it while moving it in successively larger concentric circles, starting at the center of the mortar, moving outward to the sides of the mortar, and then back again toward the center. To ensure adequate mixing and uniform particle size reduction, compacted powder is constantly removed from the sides of the mortar and the pestle by scraping with a spatula. Three different types of mortars are available for triturating drugs. Their properties and uses are described in the section on compounding equipment in Chapter 13. The process of trituration is illustrated on Color Plate 1 and is demonstrated in Section 3, Part 3 Compounding Drug Preparations, on the CD that accompanies this book.
b. Pulverization by intervention
(1) Some compounds do not lend themselves to direct trituration and must be handled in special ways. For example, some substances have hard crystalline structures that do not crush or triturate easily. The manner in which these drugs are handled depends on their ultimate use. If they are to be added to a liquid or semisolid preparation and if they are soluble in a suitable solvent, they may be dissolved first and incorporated as a solution. If they are to be included in a powdered dosage form, the procedure is more complex. One possible technique is pulverization by intervention. This process is described next.
(2) Pulverization by intervention uses recrystallization as a method of obtaining fine particles. The word intervention refers to the first step of the process, dissolving the drug in a suitable solvent, the solvent being the so-called intervening compound. In this process, the solid is first dissolved in a minimum volume of a volatile solvent such as alcohol.
(a) If the volume of the liquid is small and the rest of the powder in the preparation is not soluble in the chosen solvent, the solution may be mixed directly with the other powdered ingredients. The powders are then mixed until the solvent has completely evaporated.
(b) If the other powder ingredients are soluble in the chosen solvent, or if too much solvent is required to dissolve the drug, the solution of the drug in the solvent is spread in a thin layer on the sides of a glass mortar or on the surface of a glass ointment slab. The solvent is allowed to evaporate, and the thin film of fine, solid crystals is then scraped off the glass surface using a metal spatula. The solid can then be blended with the other ingredients in the preparation.
(3) The most common use of this technique is to obtain fine particles of camphor. Camphor is a hard, chunky solid that does not reduce to a fine powder when triturated in a mortar. When fine crystals of camphor are needed, pulverization by intervention is a useful method.

c. Levigation is the process of reducing the particle size of a solid by triturating it in a mortar or spatulating it on an ointment slab or pad with a small amount of a liquid in which the solid is not soluble. Optimally, the liquid is somewhat viscous and has a low surface tension to improve the ease of wetting the solid. Mineral oil and glycerin are examples of common levigating agents. Levigation is used most often when incorporating solid ingredients into semisolids (e.g., ointments and creams) and is discussed in more detail in Chapter 30, Semi-solids: Ointments Creams, Gels, Pastes, and Collodions. This technique is also illustrated on Color Plate 9 and is demonstrated on the CD that accompanies this book.
C. Blending
1. The goal in blending powders is to create a homogeneous mixture. This is essential for obtaining uniform doses when mixtures of solid drugs are involved.
2. Four methods—spatulation, trituration, sifting, and tumbling—are generally described in pharmacy textbooks as methods of blending in extemporaneous compounding. Compounders may also use newer technology, such as electric mixers and blenders.
a. Spatulation is the mixing of powders on an ointment slab or pad using a spatula. With this method, there is no particle size reduction, so the powders to be mixed must be fine and of uniform size. Because no pressure is used, the resulting powder is usually light and is not compacted. This method should be used when hard trituration is to be avoided, such as when blending powders that have previously been coated to prevent the formation of a liquid eutectic mixture.
b. Trituration is described earlier in this section (see V.B). It is the preferred method of blending under most circumstances because it mixes powders more intimately than other methods. It should always be used when making mixtures that contain small quantities of potent drugs. Because trituration accomplishes two processes at the same time—namely, particle size reduction and blending—this method saves time when powders of unequal particle size are being combined.
c. Clear glass or plastic bottles and zipper-sealed polyethylene bags are useful for mixing powders by tumbling. These vessels are especially useful when it is important to carefully contain the powders, such as hazardous or cytotoxic substances or lightweight powders that may get into the air when mixed using trituration or spatulation.
d. Pharmacists and pharmacy technicians who do a lot of compounding use a variety of other equipment for blending. For example, blending moderate to large quantities of powders can be accomplished efficiently with an electric mixer. Special stainless steel sifters, available from vendors of compounding equipment and supplies, can also be used for blending powders.
3. Although visual inspection of the finished powder is an important general quality control measure, visual determination of adequate mixing is usually nearly impossible. This is because most drugs are white powders, so color of the powder mixture is not a good indicator of homogeneity. The following methods are useful for ensuring proper blending of powders.
a. At the beginning of the blending process, a small amount (approximately 0.1%) of a certified dye is added to the mixture; when the dye is evenly dispersed and the color is uniform, the powder is adequately mixed.
b. A more common approach is to use well-tested techniques, such as geometric dilution or alternate addition by portions, and mixing for a length of time found by individual experience to ensure proper blending of the powders.

4. Blending techniques to ensure adequate mixing
a. Geometric dilution is used when blending two or more powder ingredients of unequal quantities. It is a method designed to help ensure that small quantities of ingredients, usually potent drugs, are uniformly distributed throughout the powder mixture. Trituration usually is the blending method of choice with geometric dilution, because it gives more intimate mixing than other methods. The steps in geometric dilution are given here, and the technique is described in the compounding procedures with Sample Prescriptions 25.2 through 25.5; it is also demonstrated on the CD that accompanies this book.
(1) Weigh all ingredients for the preparation.
(2) Place the ingredient present in the smallest quantity in a mortar.
(3) Select the ingredient present in the next largest quantity and place in the mortar an amount of this ingredient approximately equal in powder volume to that of the first ingredient.
(4) Triturate the powders well until a uniform mixture is achieved.
(5) Add a volume of powder of the second ingredient equal in size to the powder volume of the mixture in the mortar and triturate well.
(6) Continue adding powder to the mortar in this fashion, always adding a volume of powder equal to the volume of powder mixture in the mortar, until all the powder ingredients have been added.
b. When a formulation calls for combining relatively equal amounts of moderate to large quantities of two or more powdered ingredients, a uniform mixture can be obtained most easily by first combining and mixing small portions of each ingredient and then adding additional portions of each alternately, with adequate trituration or mixing with each addition. This process is sometimes referred to as alternate addition by portions.
VI. COMPOUNDING BULK POWDERS
A. Topical bulk powders
1. Topical bulk powders often contain one or more active ingredients incorporated in a diluent powder. Powders most often chosen as diluents for external bulk powders are starch and talc.
2. The techniques for preparing and mixing the powders are described in the previous section.
3. Containers for topical powders
a. Although topical powders may be dispensed in a wide-mouth bottle or container, they are much more easily applied by the patient when dispensed in a sifter-topped powder can or shaker canister. Another alternative is a plastic bottle with flip spout, snap cap, or yorker spout.
CONTAINERS FOR TOPICAL POWDERS
b. At one time, shaker canisters were made of pasteboard, and therefore these were not tight containers. Bulk powder formulations that contain volatile ingredients can lose these components through evaporation when they are dispensed in nontight containers. Furthermore, moisture from the environment can permeate through these containers and into the contained powder. Shaker containers made from hard plastic are now available, but it is important to check with the container distributor concerning the classification as either a well-closed or tight container. Bulk powders that contain either volatile active ingredients or components that are sensitive to moisture should be dispensed, when possible, in a tight container. If this is not possible or practical, a conservative beyond-use date should be assigned to the preparation. This is illustrated with Sample Prescriptions 25.2 and 25.3.
4. Labeling:The concentrations of active ingredients are expressed as percent weight-weight or as metric weight or units of active ingredient per gram of powder.
B. Bulk powders for internal use
1. Internal-use bulk powders usually do not need added diluent unless required by a particular compounding situation. (One example would be the protection of a potential eutectic mixture, which is discussed in section VIII of this chapter and in section III. B in Chapter 37.) Agents commonly used as diluents or adsorbents for internal products include magnesium carbonate, light or heavy magnesium oxide, calcium carbonate, starch, and lactose.
2. The techniques for preparing and mixing the powders are described in section V of this chapter.
3. Containers for internal-use bulk powders
a. Bulk powders for internal use are dispensed in wide-mouth powder squares, pharmaceutical rounds, or other wide-mouth containers. When the dose to be administered is a teaspoonful or tablespoonful, the container selected should, when possible, allow the patient or caregiver to insert the measuring spoon to withdraw the appropriate dose.
b. As with external bulk powders, the nature of the powder components should be considered when selecting a container, and tight containers should always be used when the situation dictates.
4. Labeling: Internal bulk powders are labeled with the weight of active ingredient per volume to be ingested or administered (e.g., teaspoonful, tablespoonful). To determine the content of active ingredients in this volume, the pharmacist must perform the following procedure:
a. Prepare the formulation as directed in the prescription order.
b. Using the appropriate measuring device (e.g., teaspoon), measure the volume of powder to be taken or administered.
c. Weigh this volume of powder.
d. From the concentration or percent of active ingredient(s) in the prepared powder and the weight of the volume to be administered, calculate the weight(s) of active ingredient(s) in the volume to be administered. An example of this procedure is given in Sample Prescription 25.1.
VII. COMPOUNDING DIVIDED POWDERS OR CHARTULAE
A. Divided powders: Also known as chartulae or powder papers, divided powders have individual doses of powder packaged in folded papers or plastic bags.
1. Amount of powder
a. Prepare enough powder for one extra dosage unit, because some powder will be lost in the blending process. If the prescription contains a controlled substance, this loss in compounding must be minimal and should be documented on the prescription order or compounding record sheet.
b. If the amount of powder for each packet is less than the minimum weighable quantity (MWQ) for the balance being used, an inert diluent powder must be added. This procedure is illustrated in Sample Prescriptions 25.4 and 25.5 in this chapter.
c. If the amount of powder is above the MWQ but is still small (e.g., less than 300 mg per packet), a diluent such as lactose may be added to bring the quantity of powder per packet to an amount that is convenient for handling and administration. This is illustrated with Sample Prescription 25.6.
(1) An intermediate amount of powder (200 mg to 500 mg) per packet is desirable.
(2) Smaller quantities are difficult to handle, and any amount left in the powder paper or bag or spilled by the patient or caregiver significantly affects the dose. In a study, nifedipine divided powders were compounded using crushed nifedipine tablets and lactose as a diluent. Each packet was formulated to contain 1 mg of nifedipine in 500 mg of powder. An analysis showed that the delivered content was 0.92 mg nifedipine per packet and that three-fourths of the loss was found on the powder papers (5). Obviously, the loss of active ingredient would have been greater if the drug had not been diluted with lactose.
(3) Large quantities of diluent should also be avoided, since larger amounts of powder are more difficult to mix into liquid vehicle or soft food for administration.
2. The techniques for preparing and mixing the powders are described previously in section V of this chapter.
C. Packaging the divided powders
1. Divided powders can be folded into powder papers or packaged in reclosable, so-called zipper-lock polybags or heat-sealed in polyethylene or cellophane bags.
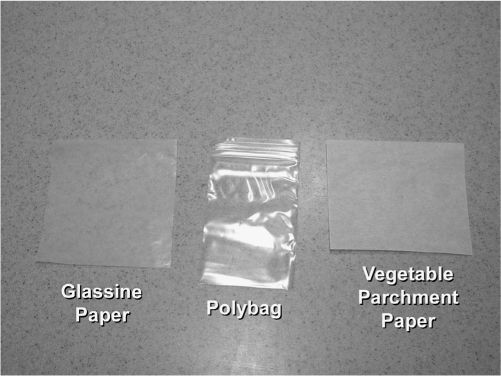
POLYBAGS AND POWDER PAPERS
2. Polybags
a. Polybags are available in various sizes and can be purchased either with reclosable zipper-type seals or as bags that require a heat-seal or adhesive tape seal. Amber or opaque polybags are also available for drugs that require protection from light.
b. Preparing divided powders with polybags is quite easy.
(1) The weighed quantity of prepared powder for one dose is placed in each bag.
(2) For reclosable bags, the closure is zipped shut. Plain polybags may be sealed by tape or heat-sealed with an impulse sealer.
(3) The sealed bags may be dispensed in a hinged or telescoping box or may be placed in a powder square or other tight container with a screw top.
3. Folded powder papers
a. Folded powder papers are used only when other packaging materials for divided powders are not available. Folded powder papers have the following disadvantages over divided powders packaged in polybags: time-consuming preparation, poor moisture barriers, and failure to meet safety-packaging regulations.
b. Types of paper most commonly used for folded powder papers include the following:
(1) Glassine weighing papers, as described in section III. D. in Chapter 14.
(2) Powder papers made of vegetable parchment paper, available in a variety of sizes ranging from 2½″ × 3¾″ to 4½″ × 6½″.

c. Folding papers for divided powders is demonstrated on the CD that accompanies this book.
D. Use of tablets and capsules in divided powders
One of the main uses of divided powders is for supplying doses for patients who cannot swallow whole tablets or capsules.
1. Always make certain that the manufactured dosage form is one that may be crushed. The topic of oral solid dosage forms that should not be crushed is discussed in section II. C. in Chapter 13.
2. If the dose needed is a whole unit of a tablet or capsule, there are some convenient alternatives to making divided powders.
a. For capsules, the powder can be emptied easily from the capsule shell by the patient or caregiver.
b. Because tablets need to be crushed, other methods are required. Tablet crushers, intended for use by patients, nurses, or other caregivers, are available at various prices, ranging from less than $10 to deluxe models that cost more than $50. One easy, inexpensive method is for the patient or caregiver to place the tablet in a small plastic bag, such as a sandwich bag, and crush the tablet with the back of a spoon.

TABLET CRUSHER
3.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree