OUTLINE
Preparation of Parenteral Dosage Forms
Disadvantages of Parenteral Therapy
Parenteral Routes of Administration
Calculations for Parenteral Preparations and Administration
I. INTRODUCTION
Although most parenteral products are manufactured by the pharmaceutical industry, many pharmacists and pharmacy technicians—particularly those working in hospitals, clinics, or home health care practices or those servicing long-term care facilities—routinely handle and manipulate IV admixtures and injections. As was indicated previously, these pharmacy personnel require special knowledge and training. Most often, this begins in pharmacy school or technician training courses, but there is also on-the-job training plus elective courses, video and multimedia resources, special seminars, and much published literature on this subject. Such training is described in USP Chapter 〈797〉, Pharmaceutical Compounding—Sterile Preparations, as outlined in Chapter 32. Various professional organizations, such as the American Society of Health-System Pharmacists, National Home Infusion Association, and the National Association of Boards of Pharmacy, to name a few, have developed training materials and models for practice. A pharmacist practicing in this specialty area has a special responsibility for knowing and understanding the standards of practice for handling sterile drug products, as well as understanding the physiologic requirements of infusing medications safely. Sample medication orders are presented in this chapter under the presumption that the pharmacist or technician has the necessary training and experience to ensure that the principles of sterile technique presented in Chapter 32 are scrupulously followed.
II. PREPARATION OF PARENTERAL DOSAGE FORMS
The procedures given here are very basic. Anyone compounding sterile preparations requires advanced instruction using the resources described previously.
A. Review the order for legibility and safety, including the following:
1. Clarification of unacceptable abbreviations.
2. Verification of completeness of information necessary to perform dose calculations.
B. Check the dose, including allowable
1. Route of administration.
3. Concentration of the solution.
4. Rate of administration.
A later section (V) on parenteral routes of administration gives guidance on some on these factors.
C. Check for stability and compatibility of the preparation. Verify the type of venous access (peripheral, central line, peripherally inserted central catheter, etc.) to ensure that the osmolality of the infusate is within acceptable ranges.
D. Perform any additional necessary calculations. Verify the correctness of all calculations performed by other health care professionals in supplying the order to the pharmacy.
E. Prepare the appropriate labels.
F. Remove any personal items known to increase particulate matter or to harbor microorganisms, such as jewelry, cosmetics, artificial nails, hats, and sweaters.
G. As previously described in Chapter 32, before entering the buffer area, perform body cleansing and don appropriate garb in order from dirtiest to cleanest areas as follows: (i) shoe covers, (ii) hair and beard covers, and (iii) eye shields.
H. Wash hands, fingernails, and forearms up to the elbows with an antimicrobial agent such as is described in the Center for Disease Control and Prevention Guideline for Hand Hygiene in Health-care Settings (1).
I. Put on a nonshedding gown or chemoprotective gown as appropriate.
J. Enter the buffer zone. Disinfect hands with an alcohol-based surgical scrub demonstrating persistent activity. Allow hands to dry completely. Put on sterile gloves, being sure to cover the cuff of the gown so that no skin is exposed.
K. Disinfect the direct compounding area (DCA) using materials, techniques, and frequency as described in Chapter 32 and USP Chapter 〈797〉.
L. Assemble the supplies needed to make the preparation and disinfect any surfaces as directed in Chapter 32 and USP Chapter 〈797〉. This includes, but is not limited to, syringes, needles, drug products, IV solutions, and alcohol swabs.
M. Prepare the preparation, applying appropriate principles of sterile technique.
1. If using a horizontal laminar airflow workbench (LAFW), be sure to work a minimum of 6” inside the front edge of the hood.
2. If using a biological safety cabinet (BSC), work behind the front shield and above the area where the laminar airstream splits to enter the grills at the front and back edges of the work surface that allow the air to recirculate.
3. If using a compounding aseptic isolator (CAI) or a compounding aseptic containment isolator (CACI), follow the manufacturer’s guidelines for use and training materials.
4. Be careful not to let anything come between a critical surface and the air coming from the high-efficiency particulate air (HEPA) filter. Be aware of placement of supplies and zones of turbulence created by articles in the laminar airflow stream.
5. Be careful not to touch any critical surfaces such as the shaft or other parts of the needle except its cap, or the syringe tip or any part of the syringe plunger except the disk or lip that is used to move the plunger.
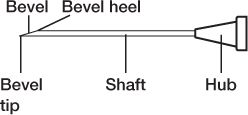
PARTS OF A NEEDLE
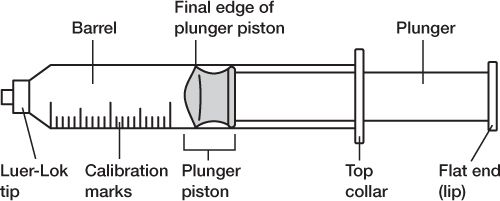
PARTS OF A SYRINGE
6. Wipe all vials, ampuls, and injection ports with sterile alcohol wipes.
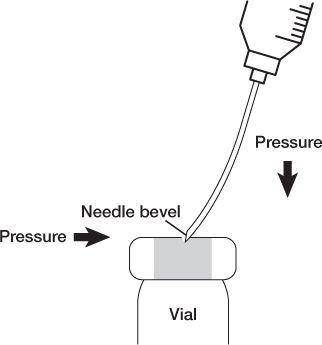
a. Swab the diaphragms of vials from back to front before entering with a needle.
b. Enter vial diaphragms with the needle bevel up and use slight lateral pressure so the needle tip and heel go in the same hole to prevent coring the diaphragm.
c. Remember that vials are sealed systems, so it is important to maintain equalized pressure: volume of air or liquid in = volume of liquid or air withdrawn.
NEEDLE PENETRATION OF A VIAL TO PREVENT CORING
d. When using ampuls, eliminate any possible glass shards in the liquid withdrawn by using a filter needle.
e. When injecting drug solution through the injection port into a large-volume parenteral bag or a minibag, be sure that the needle penetrates both the exterior diaphragm and the inside diaphragm. Also use care to ensure that the needle does not puncture the IV bag.
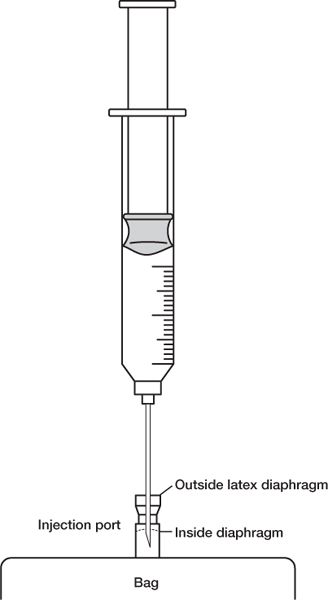
NEEDLE PENETRATION OF AN IV INJECTION PORT
N. Remove the product from the DCA and immediately apply the appropriate labels.
O. Properly dispose of sharps and other used supplies.
III. USES OF PARENTERAL PRODUCTS
A. Parenteral administration offers one alternative when a patient is unable (e.g., unconscious, vomiting) to take medication by mouth.
B. Some drugs must be given parenterally because they are not therapeutically active when taken orally owing to inactivation in the gastrointestinal tract or first-pass metabolism by the liver.
C. The parenteral route may be necessary or preferred when drug action is required immediately.
D. In some cases, a drug must be injected because it requires direct delivery to an organ, a lesion, a muscle, or a nerve.
E. Fluids, electrolytes, and/or nutrients may be delivered parenterally for patients who cannot take these orally.
F. Depots of drugs in long-acting drug delivery systems injected into muscle masses may offer superior therapy or convenience.
G. Implantable pumps offer advantages in certain circumstances.
IV. DISADVANTAGES OF PARENTERAL THERAPY
A. Manufactured parenteral products are more difficult and costly to produce than are nonsterile dosage forms. Because they must conform to strict requirements for microbiologic purity, particulate matter, and pyrogenicity, special equipment and facilities are needed.
B. In pharmacies and patient care, special equipment, devices, and techniques are also required for the safe preparation, handling, and administration of parenteral products. Specially trained personnel are needed.
C. Once administered, a parenteral product cannot be removed. Problems with drug, dose, or adverse effects may be difficult or impossible to reverse.
D. Any introduction of pathogens into the product during production, preparation, manipulation, or handling or during administration to the patient can have serious and even deadly consequences.
E. Because drug products are being injected directly into tissue, pain or tissue damage may be associated with the administration.
V. PARENTERAL ROUTES OF ADMINISTRATION
Note: The size of a needle is designated using two numbers, the length in inches of the shaft and the gauge, which is the diameter of the needle bore.
A. Intradermal (ID)
1. Injection area: located just below the surface of the skin (at the interface between the epidermis and dermis). This route is most often used for skin tests in which systemic absorption is undesirable and could be dangerous (e.g., serious allergic reactions).
2. Volumes: limited to small quantities, usually 0.1 mL, but may be as small as 0.02 mL and as large as 0.5 mL.
3. Syringe size: 1-mL syringes, often labeled tuberculin, because these syringes were used to administer tuberculin skin tests. Unlike other syringes, these are available with and without needles attached. If a syringe is sent to a nursing unit with a needle attached, you must be certain the needle cover is snapped securely in place. If it is not sent with a needle, the syringe should be filled with 0.1 mL excess for priming the new needle. The syringe should then be labeled with a statement to this effect:“This syringe contains 0.1 mL excess for priming.”
4. Needle sizes: 25 to 30 gauge, ⅜ to ⅝ inch long.
B. Subcutaneous(subcut or subcutaneous; see information on error-prone abbreviations in Table 1.1 of Chapter 1)
1. Injection area: subcutaneous fat tissue located beneath the skin between the dermis and muscle. When administering a drug subcutaneously, the skin may be pinched up to avoid giving the drug into the muscle. This route is used for insulin, injectable pain medication, and others where specified.
2. Volumes: limited to approximately 2.5 mL (these upper limits may be painful and the preparation must be injected over 1 to 5 minutes). For continuous subcutaneous infusions, the maximum volume per infusion site has been reported in various sources to be between 3 and 10 mL/hr depending on the therapy being delivered to the tissue. For example, in the text Cancer Nursing: Principles and Practice, infusion of opioid analgesics by subcutaneous infusion is recommended at rates not exceeding 5 to 10 mL/hr (2).
3. Syringes sizes: 1 or 3 mL.
4. Needle sizes: depends on use. Insulin syringes have ultrafine needles of 30 gauge (a 32-gauge needle is now available), ½ inch;“hypos” are often 25-gauge, ½ to ⅝ inch long. Insulin syringes come with the needle attached. It is left in place for administering the dose after withdrawing the drug from the product vial. For other drugs, the needle used for withdrawing the dose is usually removed, a Luer tip cap is applied, and the nurse or caregiver selects and attaches an appropriate needle at the time of injection. In this case, excess for priming the new needle should be included in the syringe and the syringe labeled to this effect. For continuous infusions, several catheters are available on the market to facilitate this method of drug delivery, from 25- to 27-gauge butterfly catheters to catheters using a Teflon infusion cannula inserted at right angles into the subcutaneous tissue.
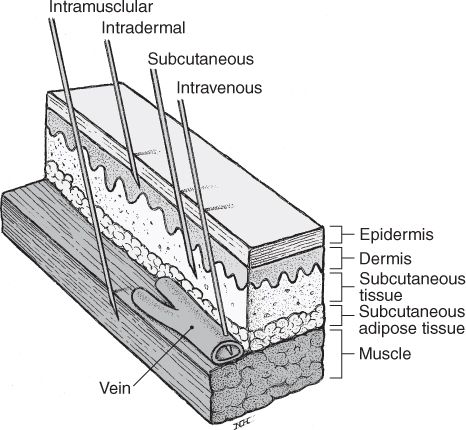
VARIOUS PARENTERAL ROUTES OF ADMINISTRATION.
(Reprinted from Stein SM. Drug administration. In: Boh LE, ed. Pharmacy practice manual: a guide to the clinical experience, 2nd edition. Philadelphia: Lippincott Williams & Wilkins, 2001.)
C. Intramuscular (IM)
1. Injection area: muscle mass—deltoid (arm), gluteus maximus (buttocks), vastus lateralis (top of leg). Any nonirritating drug can be given by this route.
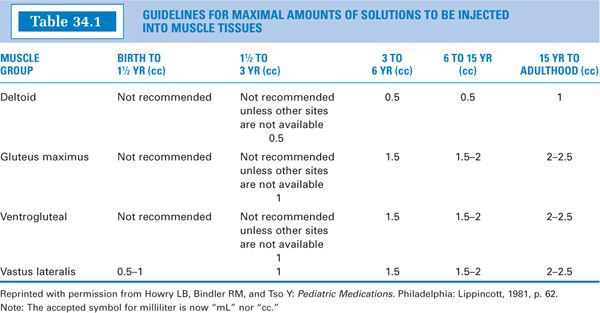
2. Volumes:The volume administered is limited by the mass of the injected muscle. For adults, up to 2 mL may be given in the deltoid muscle of the upper arm, and up to 5 mL into the gluteal medial muscle of the buttock (these upper limits may be painful). For children, the volumes are more restricted. See Table 34.1 for guidelines. Notice that for small children, the vastus lateralis muscle is the recommended muscle because it is the largest muscle mass in children younger than 3 years, and it is free of major nerves and vessels. The gluteus maximus is not well developed until a child has walked for at least 1 year (3). It is also avoided because it has a major large nerve, the sciatic nerve, running through the middle. Notice that for children up to 3 years, the maximum volume is 1 mL (3). Be sure to keep this in mind when making IM injections for children.
3. Syringes sizes: 1 to 5 mL.
4. Needle sizes: 20 to 23 gauge, ½ to 1½ inches long.
D. Intravenous (IV)
1. Injection areas: veins. This route is used for fluid, electrolyte, and nutrient replacement; for administration of any drug that needs to get into systemic circulation immediately; for irritating drugs; and for drugs that require carefully controlled blood levels.
2. Volumes: Obviously, volume is less of a limitation with IV therapy. There are fluid restrictions—approximately 3 liters per day for adults and less for children. Certain disease states further restrict fluid load. The flow rate may also be restricted by the size of the vein chosen for administering the drug.
3. Syringe sizes: 1 to 60 mL.
4. Needle: 20 to 23 gauge,1/2 to 11/2 inches long.
5. Intravenous administration is further subdivided into continuous or constant infusion, intermittent infusion, and bolus or IV push. For calculations involving flow rate for continuous or intermittent infusions, see the next section (VI) on calculations.
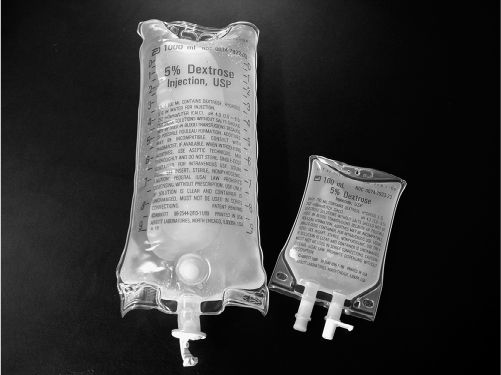
PICTURE OF LARGE-VOLUME PARENTERAL (LVP) AND SMALL-VOLUME PARENTERAL (MINIBAG)
a. Continuous: The drug is added to a large-volume parenteral (LVP) solution, and the solution is then slowly and continuously dripped into a vein.
(1) Advantages
(a) It allows fluid and drug therapy to be administered simultaneously.
(b) It achieves continuous, constant blood levels for the drug.
(c) It minimizes vein irritation and trauma because most drugs are less irritating when in dilute solutions.
(d) Continuous infusion is usually a cost saver over intermittent or bolus administration, because fewer units are needed and less nursing and pharmacy staff time is involved in preparation, processing, and administration.
(a) IV infusion requires greater monitoring because it runs continuously or requires a centrally inserted catheter or a peripherally inserted central catheter (PICC) to ensure infusion into the large vessels of the central vasculature (e.g., superior vena cava).
(b) If the IV infiltrates and cannot be continued, part of the dose has not been administered.
(c) It must be used with caution in fluid-restricted patients.
(d) The extended run times cannot be used with certain unstable drugs.
(e) In the event of an infusion device or tubing malfunction (free-flow), there is potential for a serious adverse event if the entire contents of the solution is infused over a short time period.
b. Intermittent: The drug is added to an intermediate volume (25 to 100 mL) and given in an intermediate period of time (15 to 60 minutes), at spaced intervals, such as every 6 hours.
(1) Advantages
(a) It requires less monitoring than does continuous infusion.
(b) The complete dose is given in a moderate fluid volume and over a moderate period of time; therefore, there is less chance of toxicity than with bolus administration, without the disadvantages of continuous administration.
(c) Many drugs are more stable at moderate concentrations than in the concentrated solutions required by bolus administration.
(d) Some drugs are more effective when given by this method since they depend on high peak levels to achieve the desired outcome
(2) Disadvantages:
(a) Fluids and some electrolytes cannot be given this way.
(b) Drug blood levels are less constant than with continuous administration, which may decrease the effectiveness of some medications.
(c) The method cannot be used for direct administration to an organ or tissue.
(d) It is sometimes impractical for immediate injection in emergency situations.
c. IV push or bolus: The drug solution is placed in a syringe and administered in a short period of time (minutes) directly into a vein or IV tubing that goes into a vein. This may be a onetime administration or it may be repeated at spaced intervals.
(1) Advantages
(a) IV push or bolus can be used for immediate injection in emergency situations.
(b) It requires no ongoing monitoring of IV fluid administration.
(c) It is less expensive than intermittent administration because there is no pump, controller, extra IV tubing, or bag.
(2) Disadvantages
(a) Many drugs are more irritating in the highly concentrated solutions used for IV push or bolus.
(b) Some drugs are less stable in concentrated solutions.
(c) Drug toxicity is a greater problem when a total dose is given in a bolus over a short period of time.
(d) Drug blood levels fluctuate more than with either continuous or intermittent IV dosing.
(e) When repeated doses are given, more staff time may be required, because at least 2 to 10 minutes may be needed at the bedside for each dose given.
VI. CALCULATIONS FOR PARENTERAL PREPARATIONS AND ADMINISTRATION
A. For calculations of a general nature, see Chapters 7 through 11 on pharmaceutical calculations. Calculations specific to parenteral preparations and sample medication orders are given in the pages that follow.
B. Powder volume
Some parenteral products have limited stability when in solution and are furnished by the product manufacturer as dry powder for reconstitution. At the time the drug is to be administered, a sterile diluent, usually Sterile Water for Injection or Sodium Chloride for Injection, is added.
The volume that the powder occupies after it is dissolved in solution is called the powder volume. For some drug products, this volume is so small that it is considered negligible no matter what the dilution. For other products, it occupies an intermediate volume, in which case the powder volume must be considered when making calculations involving concentrated solutions but may be ignored with more dilute solutions. For other products, the powder volume is substantial and must always be taken into account.
Although we usually think of powder volume in reference to parenteral products, all powders occupy a volume when dissolved. This is probably most obvious in the oral antibiotic powders for reconstitution. For example, a 100-mL bottle of amoxicillin for oral suspension requires the addition of 60 mL of Purified Water to give 100 mL of a suspension. In this case, the powder volume is 40 mL (100 mL − 60 mL = 40 mL). This information can be very useful. Suppose you wanted to give a dose of 165 mg for a product that has a concentration of 250 mg/5 mL when the product is reconstituted as directed on the bottle or package insert; the volume of the 165-mg dose would be:
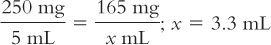
If the pharmacist or prescriber thought that this volume would be difficult for the patient to measure and that a 1-teaspoonful (5-mL) volume would be more convenient, the product could be reconstituted to give 165 mg/5 mL. This could be done in the following manner:
1. Calculate the total number of milligrams of amoxicillin in the bottle:
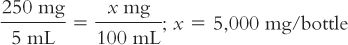
2. Calculate the number of milliliters needed for this amount of drug to give a concentration of 165 mg/5 mL:
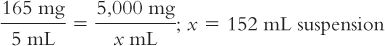
3. Based on a powder volume of 40 mL, calculate the number of milliliters of Purified Water that must be added to the bottle to give a final volume of 152 mL:
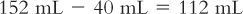
4. It may be necessary to add the water in two steps, and the partially reconstituted product will probably have to be transferred to a larger bottle to accommodate the total volume of 152 mL.
These same principles may be applied to parenteral powders for reconstitution. The following example illustrates the use of powder volume in a therapeutic situation.
Rocephin (ceftriaxone sodium) for Injection is available in 2-g vials for reconstitution. The product package insert states that when 7.2 mL of Sterile Water for Injection is added to this vial, the concentration of the resulting solution is 250 mg/mL. Your medical team wants to give this drug to a 10-month-old, 20-lb child. A drug information reference gives the following dosage information for infants and children: For serious infections (other than meningitis) in children 12 years of age or younger, the recommended dose is 50 to 75 mg/kg/day in divided doses every 12 hours.
1. The medical team is considering giving 75 mg/kg/day IM in two divided doses. The dose in milligrams of ceftriaxone is first calculated for this child.
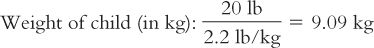
Based on this weight, the dose for this child is calculated:
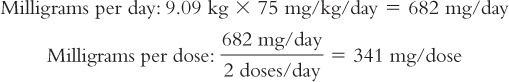
2. Next, the number of milliliters of Ceftriaxone for Injection reconstituted as recommended in the product package insert is calculated:
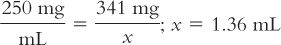
3. It is decided that this volume is too large to give IM to this child (see Table 34.1
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree