OUTLINE
Desirable Properties of Ointment Bases
Classification and Characteristics of Ointment Bases
Ingredients for Ointment Bases
I. INTRODUCTION
The purpose of this chapter is to give you basic knowledge about ointment bases and their ingredients. This information is important for two reasons: First, it will help you in guiding prescribers and patients in selecting topical products (e.g., answering the question, which is better for this purpose, an ointment or a cream?). Second, knowledge of the properties of ointment base classes and their ingredients is essential to working successfully with these ingredients when compounding with them and when developing formulations with specific properties.
In learning about ointment bases, you first need to know some definitions and terminology. Second, ointment bases have conveniently been grouped into classes that generally define their properties; knowledge about these class traits helps greatly in selecting an appropriate base for a particular use. Finally, it is useful to know something about the specific properties of ointment base ingredients; it will help you in understanding the labels of ointment products, and specific information such as solubility, melting point, and other properties will aid in selecting and using these ingredients. The ingredient section is meant as a resource for this purpose.
II. DEFINITIONS
Definitions and nomenclature for pharmaceutical dosage forms are currently in transition between use of traditional terms and definitions and a more systematic approach that has been proposed to more accurately and consistently describe drug products and preparations. During this time, it is important that pharmacists and pharmacy technicians know the traditional terms but also understand proposed definitions and nomenclature. The rationale for the changes and the development of the proposed system are discussed at the beginning of Chapter 27, Solutions, and the comparison of nomenclature and definitions specific to semisolids such as ointments, creams, gels, and pastes is presented at the beginning of Chapter 30, Semisolids: Ointments, Creams, Gels, Pastes, and Collodions. Those definitions needed for understanding the information in this chapter follow. For additional information on this subject, consult Chapters 27 and 30.
1. Traditionally the term ointment has been used for (i) the general class name for all external-use semisolids and (ii) the subclass, oleaginous semisolids. For example, USP 31 Chapter 〈1151〉 defines ointments very generally as “semisolid preparations intended for external application to the skin or mucous membranes” (1). However, pharmaceutical manufacturers use the word ointment more specifically to indicate that a drug is incorporated into an oleaginous ointment base; for example, the name Hydrocortisone Ointment means that hydrocortisone is incorporated into an oil-type semisolid base.
2. Under the proposed nomenclature, this situation would be clarified; the term semisolid would be used for naming the general class, and the term ointment would be redefined more narrowly as “a viscous oleaginous or polymeric semisolid dosage form” (2), which is consistent with current usage by the pharmaceutical industry.
3. According to the USP Chapter 〈1151〉, there are four general classes of ointment (i.e., semi-solid) bases (1). These are listed here and are described in more detail in Chapter 〈1151〉 and in section III of this chapter.
a. Hydrocarbon
b. Absorption
c. Water-removable
d. Water-soluble
4. Within these classes, the following ointment bases are listed in the excipient table at the front of the NF (3):
Caprylocaproyl Polyoxylglycerides; Diethylene Glycol Monoethyl Ether; Lanolin, Lauroyl Polyoxylglycerides; Linoleoyl Polyoxylglycerides; Hydrophilic Ointment;White Ointment;Yellow Ointment; Oleoyl Polyoxylglycerides; Polyethylene Glycol Monomethyl Ether; Petrolatum; Hydrophilic Petrolatum;White Petrolatum; Rose Water Ointment; Squalane; Stearoyl Polyoxylglycerides;Type II Vegetable Oil
B. Cream
1. Although creams meet the general definition of an ointment, they have been given a separate section in USP 31 Chapter 〈1151〉. This section has more of a historical description of this term than a specific definition. Although it states that creams are “semisolid dosage forms containing one or more drug substances dissolved or dispersed in a suitable base” (1), it then discusses the evolution of this term to include or exclude certain types of semisolid emulsions and aqueous microcrystalline dispersions.
2. The proposed new nomenclature both simplifies and clarifies the situation by defining a cream as “a dosage form comprising a viscous semisolid emulsion” (2). Under this definition, creams would fall into two of the four general ointment base classes listed earlier: both water-containing absorption bases and water-removable bases.
C. Paste
1. As with creams, pastes meet the general definition of an ointment, but they have been given a separate section in USP 31 Chapter 〈1151〉. They are defined as “semisolid dosage forms that contain one or more drug substances intended for topical application” (1). Then, to more clearly distinguish a paste from other topical semisolids, Chapter 〈1151〉 lists two classes of pastes and gives an example of each. One group consists of very stiff ointments with a high concentration of solid particles in an oleaginous base: Zinc Oxide Paste USP is an example of this group. The other subclass is also very thick but has a single aqueous phase with a high polymer content: Carboxymethylcellulose Sodium Paste is an example of this group (2).
2. The proposed new nomenclature defines a paste as “a semisolid preparation with a stiff consistency containing a relatively high concentration of solids” (2).
D. Gel
1. Many, but not all, gels fit within the Chapter 〈1151〉 general definition of an ointment; some would be considered thick suspensions rather than semisolids, and some are for oral rather than topical administration. As with creams and pastes, gels are classified separately in Chapter 〈1151〉 and are defined there as “semisolid systems consisting of either suspensions made up of small inorganic particles or large organic molecules interpenetrated by liquid” (1). The proposed definition is quite similar: “a dispersion of small inorganic particles or a solution of large organic molecules rendered jellylike in consistency” (2).
2. The gels that are thick suspensions of small inorganic particles are systems such as Aluminum Hydroxide Gel USP. Some are called magmas if the size of the dispersed phase is large (e.g., Bentonite Magma) (1). These gels must be labeled “Shake before use.” Some are thixotropic, forming semisolids on standing but becoming a liquid when shaken.
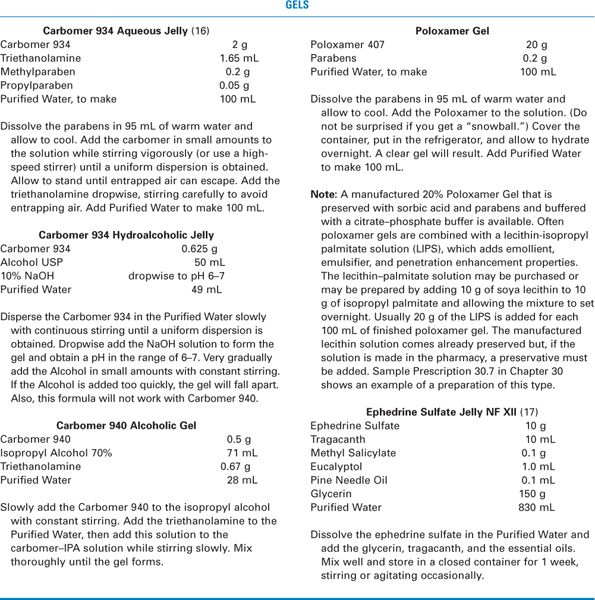
3. The gels that have a more jelly-like consistency have large organic polymer molecules like carbomer, methylcellulose, and poloxamer dispersed in a liquid, usually water or a hydroalcoholic solution. An example of this type of gel is Hydrocortisone Gel USP, which is hydrocortisone in a hydroalcoholic gel base (4).
E. Emollient: An agent that softens the skin or soothes irritation in skin or mucous membrane.
F. Protective: A substance that protects injured or exposed skin surfaces from harmful or annoying stimuli.
G. Occlusive: A substance that promotes retention of water in the skin by forming a hydrophobic barrier that prevents evaporation of moisture from within the skin.
H. Humectant: A substance that causes water to be retained because of its hygroscopic properties.
III. DESIRABLE PROPERTIES OF OINTMENT BASES
Certain properties are desired for all ointment bases, no matter what their particular use. These include the following:
A. Chemically and physically stable under normal conditions of use and storage
B. Nonreactive and compatible with a wide variety of drugs and auxiliary agents
C. Free from objectionable odor
D. Nontoxic, nonsensitizing, and nonirritating
E. Aesthetically appealing, easy to apply, and nongreasy
F. Remains in contact with the skin until removal is desired, then is removed easily
IV. CLASSIFICATION AND CHARACTERISTICS OF OINTMENT BASES
A. Many factors determine the choice of an ointment base. These include the action desired, the nature of the medication to be incorporated and its bioavailability and stability, and the desired shelf life of the finished product (1). The choice of a particular base matches these factors with the properties of an ointment base class.
B. Ointment Bases
As stated previously, the USP recognizes four general classes of ointment bases to be used therapeutically or as vehicles for active ingredients (1). Each has very specific and unique characteristics (5,6). As noted in the previous chapters of Part 4, this text employs the usual convention of using upper-case first letters for words designating offical USP-NF articles (e.g., Alcohol, Purified Water) and lower-case first letters for words designating the chemical substance (e.g., ethanol, water).
1. Hydrocarbon or oleaginous bases
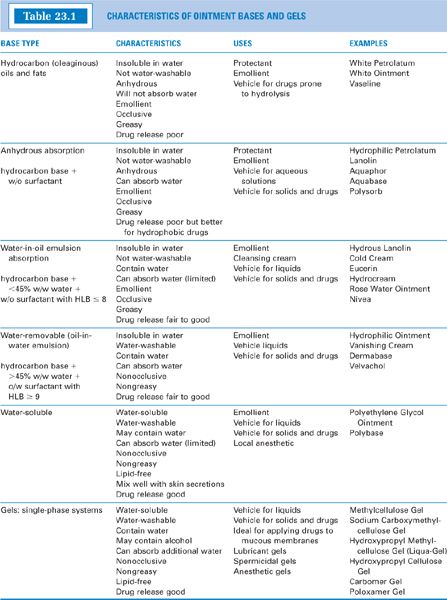
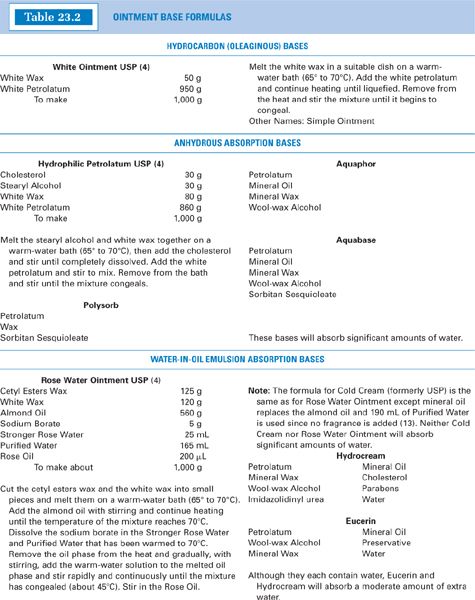
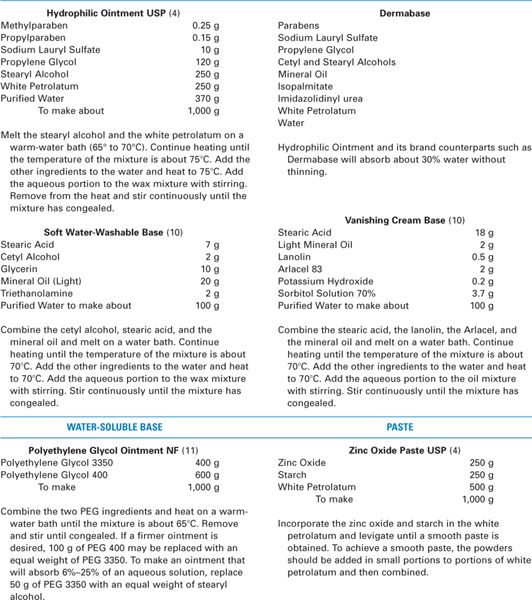
a. See Table 23.1 for characteristics and examples of these bases; see Table 23.2 for some sample formulas.
b. Advantages
(1) Inexpensive
(2) Nonreactive
(3) Nonirritating
(4) Good emollient, protective, and occlusive properties
(5) Not water-washable so they stay on the skin and keep incorporated medications in contact with the skin
c. Disadvantages
(1) These bases have poor patient acceptance because of their greasy nature.
(2) They are not removed easily with washing when this is desired (Note: may be removed using mineral oil, which is then washed off with soap and warm water).
(3) They cannot absorb water and can absorb only limited amounts of alcoholic solutions, so most liquid ingredients are difficult to incorporate into hydrocarbon bases. Possible strategies for dealing with this difficulty are discussed in Chapter 30, Semisolids: Ointments, Creams, Gels, Pastes, and Collodions, and are illustrated with Sample Prescriptions 30.4 and 30.5.
(4) Because these bases do not absorb or mix with aqueous solutions, aqueous skin secretions do not readily dissipate.
2. Absorption bases
a. See Table 23.1 for characteristics and examples of these bases; see Table 23.2 for some sample formulas.
b. Absorption bases have two subgroups:
(1) Anhydrous absorption bases
These are hydrocarbon bases that contain an emulsifier or emulsifiers that form water-in-oil emulsions when water or an aqueous solution is added.
(2) Water-in-oil emulsions
These are absorption bases that contain water, the amount depending on the base. As semi-solid emulsions, they are classified as creams under the proposed nomenclature scheme.
c. Advantages
(1) Absorption bases have moderately good protective, occlusive, and emollient properties.
(2) They do not wash off easily so they hold incorporated medications in contact with the skin.
(3) They can absorb liquids.
(a) Anhydrous absorption bases can absorb significant amounts of water and moderate amounts of alcoholic solutions. This is illustrated with Sample Prescription 30.4 in Chapter 30.
(b) Because they already contain water, emulsion absorption bases absorb variable amounts of water and/or alcohol.
(4) Some lanolin-types have compositions somewhat like the sebaceous secretions of the skin. These are thought to have superior emollient properties. Martindale: The Extra Pharmacopoeia states that wool-fat preparations mixed with suitable vegetable oils or with petrolatum give emollient ointments that penetrate the skin and enhance absorption (7,8).
d. Disadvantages
(1) Some bases in this group have poor patient acceptance.
(a) The anhydrous absorption bases have a greasy nature similar to that of hydrocarbon bases.
(b) Some lanolin-type bases are somewhat sticky and have a mildly unpleasant odor.
(2) They are not easily removed with washing. (Note: As with hydrocarbon bases, they may be removed using mineral oil.)
(3) Those bases containing wool wax or wool-wax alcohols may be sensitizing. Efforts have been made to remove offending principles, including detergents and natural free fatty alcohols, which is reported to reduce the incidence of hypersensitivity to almost zero (9).
(4) Those bases with soap-type emulsifiers (e.g., Cold Cream, Rose Water Ointment) can have the compatibility problems associated with this type of emulsifying agent. This is discussed in the section on soft soaps in Chapter 20, Surfactants and Emulsifying Agents.
(5) Those that contain water may have chemical stability problems with ingredients that are sensitive to hydrolysis.
(6) Those containing water are also subject to microbial growth, and the USP requires that these contain a preservative (1).
3. Water-removable bases
a. See Table 23.1 for characteristics and examples of these bases; see Table 23.2 for some sample formulas.
b.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree