OUTLINE
Labels for Outpatient Prescription Orders
Labels for Inpatient Drug Orders
Labels for Compounded Drug Preparations
I. DEFINITIONS
A. The terms “labeling” and “label” are defined differently for manufactured drug products and for products dispensed by the pharmacist to a patient on a prescription order.
B. Labeling and labels for manufactured drug products
1. For manufactured drug products, both the Federal Food, Drug, and Cosmetic Act and the United States Pharmacopeia (USP) define and differentiate the terms “labeling” and “label” as follows. The term “labeling” is used for all labels and other written, printed, or graphic matter both upon the immediate container of a drug product or in any package or wrapper in which it is enclosed, except any outer shipping container. The term “label” designates just that part of the labeling that appears upon the immediate container (1,2).
2. Labeling and labels of manufactured drug products are strictly controlled by the U.S. Food and Drug Administration (FDA).
C. Labeling and labels for dispensed drug products
1. The NABP Model State Pharmacy Act defines the terms “label” and “labeling” for the purpose of pharmacist dispensing of drug products to patients as follows:
a. Label: “A display of written, printed, or graphic matter upon the immediate container of any Drug or Device” (3).
b. Labeling: “The process of preparing and affixing a label to any Drug container exclusive, however, of the Labeling by a Manufacturer, packer, or Distributor of a Non-Prescription Drug or commercially packaged Legend Drug or Device” (3).
2. The information on the labels of prescription drug containers dispensed to ambulatory patients is regulated by both state and federal laws. These regulations also apply to drug containers dispensed to inpatients who will self-administer their drugs.
3. Information on labels for drug products that will be administered by health professionals, such as physicians or nurses, to inpatients in such facilities as hospitals and nursing homes is not specified in federal law. At one time, this label information was also not regulated by state laws; rather, various health care organizations recommended label and labeling standards for inpatient drugs. More recently, the NABP’s Model Rules for the Practice of Pharmacy have recommended that state laws specify the required elements for labels on drug products for inpatients. Consult the applicable state laws for your practice area for regulations on dispensed drug product labels for inpatients.
II. LABELS FOR OUTPATIENT PRESCRIPTION ORDERS

A. Required information on labels for outpatient prescriptions
1. The Federal Food, Drug, and Cosmetic Act requires that drug products dispensed on prescription order be labeled with “the name and the address of the dispenser, the serial number and the date of the prescription or of its filling, the name of the prescriber, and, if stated in the prescription, the name of the patient, and directions for use and cautionary statements, if any, contained in such prescription” (4).
2. State laws have additional requirements for outpatient prescription labels. The pharmacist must label the dispensed drug product with those items specified in the federal law plus any additional state requirements.
3. As with prescription drug orders, the NABP Model Rules for the Practice of Pharmacy contain recommendations for state statutes on required items of information for the prescription product label (5). These are listed below and are illustrated in the label examples shown in Figures 2.1 and 2.2. The labels correspond to the prescription orders given in Figures 1.1 and 1.2 of Chapter 1. Additional samples are given on the CD that accompanies this book and in the prescription examples in the dosage forms chapters of the book. For the specific standards for your practice site, consult the applicable state law.
a. Name and address of the pharmacy that dispensed the drug
b. Name of the patient; if the patient is an animal, the species of animal and the owner’s name
c. Name of the prescriber
d. Directions for use as given on the prescription order
e. Date dispensed
f. Cautionary statements, if any
g. Serial number of prescription
h. Name or initial of the dispensing pharmacist
i. Name (proprietary or generic) and strength of drug product dispensed; special requirements with regard to the name of the product may apply if an equivalent drug product is dispensed (i.e., generic substitution for a branded product)
j. Name of the manufacturer or distributor of the product dispensed
k. The beyond-use date of the product (5)
FIGURE 2.1. PRESCRIPTION LABEL FOR A GENERIC DRUG PRODUCT.
FIGURE 2.2. PRESCRIPTION LABEL FOR A BRANDED, CONTROLLED SUBSTANCE DRUG PRODUCT.
Note: Some dispensing software automatically puts a 1-year beyond-use date on the label. The pharmacist or pharmacy technician must verify that this date is within the expiration date given on the container of the product dispensed. For more information on expiration and beyond-use dates, see Chapter 4 of this book.
4. Although most of the previously mentioned elements are fairly standard from state to state, there is considerable variation in state regulations governing the required label information when dispensing equivalent drug products (i.e., generic substitution for a branded or proprietary product). The NABP’s Model Rules recommend the use of the word “INTERCHANGE” or the letters “IC” on labels when an equivalent product is dispensed (5). Some states require just the generic name or the name given the generic product by the generic manufacturer or distributor; other states want words to the effect of “generic name equivalent to brand name.”This is an attempt to inform the patient without infringing on company trademarks, such as brand names. It would not be legal to label one company’s product with another company’s brand name.
5. For controlled substances in Schedules II through IV, the following auxiliary label is required: “Caution: Federal law prohibits the transfer of this drug to any person other than the patient for whom it was prescribed.”
6. Some states have additional requirements for the label. Examples include quantity dispensed, number of refills, and date of the original prescription drug order. It is the duty of each pharmacist to be informed about the specific legal requirements for labels on dispensed drug products for the state in which he or she is practicing.
B. Computerized dispensing software, created for a national market, is now used extensively for generating prescription labels. Although label software may include additional nonrequired information, it is important that pharmacists ensure that, at a minimum, all label elements required by their state law are included in the dispensing software package purchased for their practice.
C. Three of the labeling requirements merit additional discussion: (i) directions for use, (ii) name of the product/dosage form/ingredients, and (iii) strength or quantity of product or ingredients.
1. Directions for use
a. The directions for use should be written in clear, concise English using terminology that the general public (including patients with minimal education) understands. Avoid such abbreviations as BP, GI, and SOB and medical terms that the patient may not understand. For patients who do not speak English, be certain that they understand how to use their medication. Some dispensing software programs have options for labels in languages (e.g., Spanish) in addition to English. For complex dosage forms, such as inhalers, use a combination of the standard dispensing label, printed product instructions, with picture illustrations if available, and, when appropriate, demonstration of dosing techniques by the pharmacist. For more information on this topic, see Chapter 6 of this book.
b. Although the directions for use should be as close as possible to those on the prescription order, the pharmacist may—and in fact should—clarify indefinite instructions as long as the intent of the prescriber is not changed.
c. Vague labeling instructions, such as “Take as directed” or “Take as needed,” are not sufficient directions for use. They assume that the patient was given adequate additional oral instructions and that he or she had a correct interpretation and understanding of these instructions. Furthermore, such instructions rely on both the short-term and long-term memory of the patient. If vague instructions are given on the prescription order, the pharmacist should consult the prescriber for more specific instructions (6).
d. Directions for use should be in complete sentences. The following format is a useful template for most circumstances.
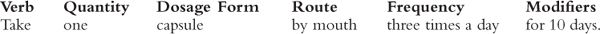
e. Verb: Use easily understood verbs, such as take, give, apply, insert, place, use, and put. Avoid outdated terms, such as “instill,” unless you are certain the patient understands. For topical administration, the adverbs “topically” or “locally” are sometimes used, but these are optional and can be confusing to the patient. For example, what does it mean to “apply locally”?
f. Quantity: Quantities are usually expressed as numbers, but they may also be descriptive adverbs, such as “lightly” or “in a thin layer.”
(1) Numbers, when part of the directions for use, should be spelled out whenever possible. This is a safety feature. Because the numbers are next to each other on the keyboard, it would be easy to type a “2” when a “3” is desired. A typing error made in spelling the number, such as tjree for three, would be detected easily, and the patient would not confuse the misspelling with another number.
(2) Fractions should also be written out, such as one-half or one-quarter rather than ½ or ¼.
(3) If numbers must be used, avoid trailing zeros (e.g., use 1 rather than 1.0) and “naked” decimal points (e.g., type 0.5 rather than .5) as described in section VI in Chapter 1.
g. Dosage form: Examples include tablets, capsules, suppositories, lotions, and drops.
(1) For oral liquids, the dosage form may be expressed as milliliters (mL) or common equivalents, such as teaspoonful or tablespoonful. Volume doses, such as teaspoons, should be written with the suffix “ful” (e.g., teaspoonful). Teaspoonfuls is the preferred plural of teaspoonful but, according to Webster’s New Collegiate Dictionary, teaspoonsful is also acceptable.
(2) If the prescription is for a bulk powder, specify “level” (e.g., level teaspoonful).
(3) To avoid dosing errors, many pharmacists will include metric volumes along with household measurement terms, such as “Take one teaspoonful (5 mL) by mouth…”
h. Route of administration: The route of administration should be specified in most cases, including eye, ear, nose, rectum, vagina, and urethra.
(1) For topical skin preparations, the route of administration is often given as “affected area.”
(2) For tablet and capsule medications given by mouth, the route is usually understood from the directions and is not required unless the prescriber has specifically written this in the directions for use. The words “by mouth” should always be included if there is any possibility of confusion. For example, the directions for use of an oral liquid antibiotic used for an ear infection should specify “by mouth” because otherwise the patient might think it should be put in the ear. Some states require the route of administration to be specified in all cases, including oral products.
(3) Specialty capsules and tablets that are used by routes of administration other than oral require specific labeling. For this reason, the USP states, “The label of any form of Capsule or Tablet intended for administration other than by swallowing intact bears a prominent indication of the manner in which it is to be used” (1).
i. Frequency:
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree




