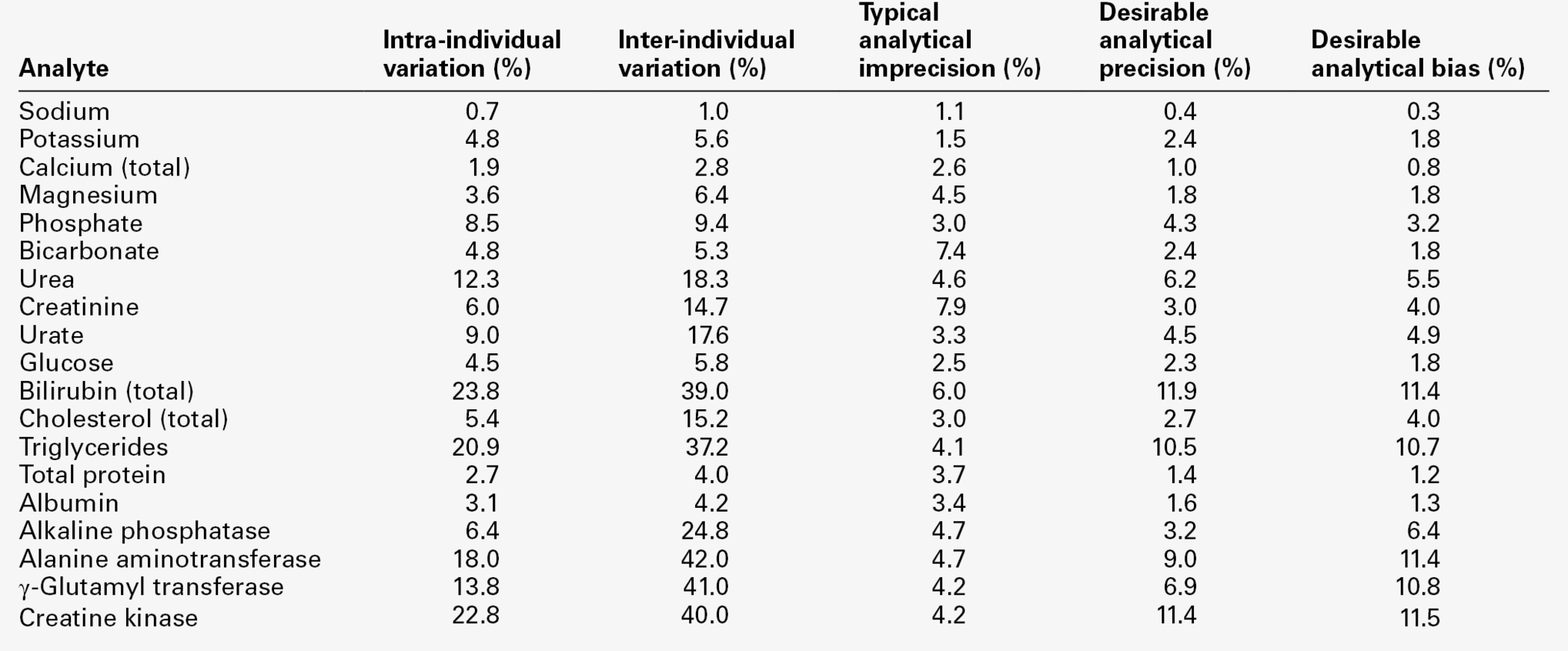
CHAPTER 2 CHAPTER OUTLINE FACTORS AFFECTING TEST RESULTS Comparison of observed results with reference limits Comparison of results with previous values Prevalence and predictive value Practical applications of the predictive value model Receiver operating characteristic curves It was emphasized in Chapter 1 that all investigations in medicine should be performed to answer specific questions. Biochemical data obtained must be considered in relation to the reason for the request, and against the background of an understanding of the relevant normal physiological and biochemical mechanisms and the way in which these respond to disease. One of the objectives of any laboratory is to ensure these data are available in a timely manner, and generated efficiently. The achievement of this goal requires careful attention to every step in the process, from the ordering of the investigation, the collection of the specimen(s) required, their transport to the laboratory and analysis, to the delivery of a report to the clinician, appropriate action being taken and the effects of this action being assessed. Amongst these many steps, the interpretation of data by clinical biochemists adds considerably to the value of the data. The workload of most laboratories is so great that it would be impossible (as well as being unnecessary) to add such comments to all reports (e.g. where the results are clearly normal). Interpretative comments (which may be individual or rule-based) are more likely to be required for more unusual tests, and for requestors who have only limited experience of the investigation in question. Typical reports requiring more detailed interpretation include those with borderline data, results that are not consistent with the clinical findings, apparently contradictory data and changes in biochemical variables during dynamic function tests. The first step in performing a biochemical investigation is for a request form to be completed, often electronically, which prompts the collection of the appropriate specimen(s) and instructs the laboratory on the investigations(s) to be performed. Depending upon the reason for the request, the expertise of the clinician and the practice of the laboratory, the request may simply be for one or more specified analyses on a body fluid; for a set (often referred to as a ‘profile’) of standard investigations (e.g. ‘thyroid function tests’); for a more involved procedure such as a dynamic function test involving the collection of serial samples following a specific stimulus, or an open request to perform whatever assays are deemed appropriate by the laboratory staff to answer the question posed in the request. The majority of biochemical test requests fall into the first two categories. The information that is required when a test is requested is summarized in Table 2.1. TABLE 2.1 Information required when requesting a biochemical investigation a In the UK, it is mandatory to supply the National Health Service Number. b It is good practice also to record the time at which the specimen and request is received in the laboratory. The generation of biochemical data is potentially subject to error at every stage in the process. It is essential that the sources of error are identified and understood, so that their effects can be minimized. The sources of errors in biochemical tests are conventionally described in three categories: • analytical: these may be random (e.g. due to the presence of an interfering substance in the specimen) or systematic (e.g. because of a bias in the method) • postanalytical: that is, occurring during data processing or transmission, or in relation to the interpretation of the data. Preanalytical factors may appear to be beyond the remit of clinical biochemists, but accreditation bodies are increasingly expecting laboratories to take responsibility for all aspects of testing. Laboratories should ensure that clinicians requesting investigations and the staff responsible for collecting the specimens understand the problems that can arise, so that specimens are collected and transported appropriately. Preanalytical factors fall into two categories: those that relate to the specimen obtained for analysis (technical factors) and those that relate directly to the patient (biological factors). These include: • correct identification of the patient • appropriate preparation of the patient where necessary • collection of the specimen into an appropriate container with the correct anticoagulant or preservative • accurate labelling of the specimen container after the sample has been collected (not before, as this carries a higher risk of a specimen being put into a container bearing another patient’s name). Primary labelling of the sample with a barcode at source reduces the risk of mislabelling in the laboratory • rapid and secure transport to the laboratory. Some specimens need to be transported under special conditions, for example arterial blood for ‘blood gases’ in a sealed syringe in an ice-water mixture; requests for blood, urine or faecal porphyrins must be protected from light. Care must be taken during specimen collection to avoid contamination (e.g. with fluid from a drip), haemolysis of blood or haemoconcentration (due to prolonged application of a tourniquet). Appropriate precautions are also required during the collection and transport of urine, faeces, spinal fluid or tissue. Specimens known to be infective (e.g. from patients carrying the hepatitis B or HIV viruses) are occasionally handled specially, but it is good practice to handle all specimens as if they were potentially hazardous. On receipt in the laboratory, the patient’s name and other identifiers on the specimen must be checked against these details on the request form, whether paper or electronic. The specimen and form should then be labelled with the same unique number. As electronic requesting becomes more common, the majority of specimens are now being labelled with a unique barcode at source. Secondary containers (e.g. aliquots from the primary tube) should also be identified with patient details and the same unique number as the primary container. Automated preanalytical robotics and track systems can minimize the number of manual interventions required during sample handling, thereby minimizing risk of errors. All these processes are facilitated if computer systems in hospitals or surgeries have electronic links to the laboratory information system. Laboratories should have written protocols (standard operating procedures) for the receipt and handling of all specimens to ensure the positive identification of specimens throughout the analytical process. Numerous factors directly related to the patient can influence biochemical variables, in addition to pathological processes. They can conveniently be divided into endogenous factors, intrinsic to the patient, and exogenous factors, which are imposed by the patient’s circumstances. They are summarized in Table 2.2. TABLE 2.2 Some biological factors affecting biochemical variables In addition, all biochemical variables show some intrinsic variation, tending to vary randomly around the typical value for the individual. The reference values (mentioned in more detail later in the chapter) for many biochemical variables do not vary significantly with age during adult life. Some, however, are different during childhood, particularly in the neonatal period. A well-known example is plasma alkaline phosphatase activity, which is higher in children, particularly during the pubertal growth spurt, than in adults. Plasma cholesterol concentrations tend to increase with age, but may fall slightly over the age of 70; plasma urate concentrations tend to rise with age. Given that renal function tends to decline with age, it might be anticipated that mean plasma creatinine concentrations would rise with age, but the tendency for loss of muscle bulk in the elderly has a balancing effect. Other age-related changes are discussed elsewhere in this book. Apart from the obvious differences in plasma gonadal hormone concentrations between adult men and women, other analytes demonstrate sex-related differences in concentration, often because their metabolism is influenced by gonadal hormones. Thus, total cholesterol concentrations tend to be higher in healthy men than in women until the menopause, after which concentrations in women tend to rise. In general, sex-related differences in biochemical variables are less between boys and girls prepubertally, while the differences between adult males and females decrease after the menopause. When age and sex are important determinants of the level of a biochemical variable, measurements in patients should be considered in relation to age- and sex-related reference values, if valid conclusions are to be drawn. Changes in many biochemical variables occur during pregnancy and, where necessary, measurements must be compared with reference values appropriate to the stage of gestation. The clinical biochemistry of pregnancy is considered in detail in Chapter 22. Plasma creatine kinase activity tends to be higher in people of sub-Saharan African descent than Caucasians (typically up to three times the upper reference limit; people of southern Asian origin may have intermediate values), but otherwise, there are no significant differences in the typical values for most biochemical variables between individuals of different ethnic origin living in the same region. Obese individuals tend to have higher plasma insulin and triglyceride concentrations than lean individuals, with an increased risk of developing type 2 diabetes and cardiovascular disease. Creatinine production is related to muscle bulk and plasma concentration may be above the usual reference range in a muscular individual, despite having a normal glomerular filtration rate. Twenty-four hour urinary excretion of many substances is greater in people of higher body mass. On the whole, however, body mass has little effect on the concentrations of substances in body fluids, although, of course, it is an important determinant of the total quantities of many substances in the body. Many exogenous factors can have profound influences on the concentrations of biochemical variables even in healthy individuals. They include the time of day, stress, posture, fasting status, drugs, exercise and concurrent illness (see Tables 2.2 and 2.3). TABLE 2.3 Some examples of in vivo effects of drugs on biochemical variables Rhythmic changes occur in many physiological functions and are reflected in changes in the levels of biochemical variables with time. The time base may be diurnal (related to the time of day, but usually nycthemeral, i.e. related to the sleep–wake cycle), catamenial (relating to the menstrual cycle) or seasonal. In addition, some hormones are secreted in sporadic bursts (e.g. growth hormone); when this occurs, it may be helpful to collect several blood samples over a short period of time and to base clinical decisions on the mean value. The best known analyte having a diurnal variation in concentration is cortisol. Its concentration is at a nadir at about midnight, rises rapidly to reach a peak at 08.00–09.00 h and then declines throughout the day. Observed values must be compared with reference values for specific times, with sampling at expected peak or trough times being the most informative. Other analytes showing a diurnal rhythm (but to lesser extents) include thyroid stimulating hormone, testosterone and prolactin. Some analytes show regular variations with a different time base. In women, during the reproductive years, the menstrual cycle is associated with regular changes in the concentrations of gonadotrophins, oestrogens and progesterone. Measurements made for diagnostic purposes must be made at the appropriate time in the cycle: for example, an increase in plasma progesterone concentration seven days before the onset of the next menstrual period is due is taken as an indication that ovulation has occurred. Plasma 25-Hydroxycholecalciferol concentration varies with the season, being higher in summer than in winter. Concern for the patient dictates that stress should be minimized at all times, but this is particularly important in relation to blood sampling for those analytes with concentrations that are responsive to (usually by increasing) stress. Pituitary and adrenal hormones are particularly affected. Thus plasma adrenocorticotropic hormone (ACTH), cortisol, prolactin, growth hormone and catecholamine concentrations all rise in response to stress. Indeed, this effect is utilized in investigations of pituitary function. However, avoidance of stress is vital when collecting specimens for the measurement of these hormones under other circumstances. Posture has a significant effect on a wide range of analytes. The best known of these are plasma renin activity and aldosterone concentration. Both are higher in the standing than the recumbent (or even sitting) position, particularly shortly after the change in posture, as a result of a decrease in renal blood flow. The effect of posture on certain other analytes is less well appreciated. When people are upright, there is a greater tendency for fluid to move from the vascular to the interstitial compartment than when they are recumbent. Small molecules and ions in solution move with water, but macromolecules and smaller moieties bound to them do not. As a result, the concentrations of proteins, including lipoproteins, and of protein-bound substances, for example thyroid and other hormones, calcium and iron, tend to be approximately 10% higher when an individual is in the upright position than when recumbent. Intermediate values occur when sitting. This effect may be relevant when values obtained from individuals when they are outpatients are compared with values obtained when they are inpatients. The concentrations of many analytes vary in relation to food intake. Frequently encountered examples include glucose, triglycerides and insulin, the plasma concentrations of which all increase following a meal, so that they should usually be measured in the fasting state, unless the effect of recent intake (as in the glucose tolerance test) is being examined. Some specific dietary constituents can affect biochemical variables; consumption of red meat in the hours before venepuncture may increase plasma creatinine concentrations by as much as 30% from fasting values. A protein-rich meal results in increased urea synthesis and increases plasma urea concentration. Long-term dietary habits can also significantly affect biochemical variables (e.g. cholesterol). The urinary excretion of many substances is highly dependent on their intake and, although some laboratories publish reference ranges for them, they are very wide. In assessing the significance of the excretion of substances in the urine, it is important to consider their intake and thus their expected excretion if renal function is normal. Thus, for example, a low urinary sodium excretion is a normal response to sodium depletion. Drugs, whether taken for therapeutic, social or other purposes, can have profound effects on the results of biochemical tests. These can be due to interactions occurring both in vivo and in vitro. In vivo interactions occur more frequently. They can be due to direct or indirect actions on physiological processes or to pathological actions. There are numerous examples in each category. Some of the better known physiological interactions are indicated in Table 2.3. Others are discussed elsewhere in this book. Pathological consequences of drug action in vivo can be idiosyncratic (unpredictable) or dose related. A measurable biochemical change may be the first indication of a harmful effect of a drug, and biochemical tests are widely used to provide an early indication of possible harmful effects of drugs, both for those in established use (e.g. measurement of serum creatine kinase activity in patients being treated with statins) and in drug trials. Drugs can also interfere with analyses in vitro (this is strictly an analytical factor, but is mentioned here for completeness), for example by inhibiting the generation of a signal or by cross-reacting with the analyte in question and giving a spuriously high signal. This field is well documented, but the introduction of new drugs means that new examples of this source of error are continually being described. Exercise can cause a transient increase in plasma potassium concentration and creatine kinase activity; the latter used to be a potential confounding factor in the diagnosis of myocardial infarction in a patient who had developed chest pain during exercise before cardiac-specific troponin measurement was introduced. Even uncomplicated surgery may cause an increase in creatine kinase as a result of muscle damage, and tissue damage during surgery can also cause transient hyperkalaemia. Major surgery and severe illness can elicit the ‘metabolic response to trauma’ (see Chapter 20), which can lead to changes in many biochemical variables. The contributions to biological variation discussed thus far are predictable, and either preventable or can be allowed for in interpreting test results. However, levels of analytes also show random variation around their homoeostatic set points. This variation contributes to the overall imprecision of measurements and must also be taken into account in the interpretation of test results. It is also relevant to the setting of goals for analytical precision (see below). Intrinsic biological variation can be measured by collecting a series of specimens from a small group of comparable individuals over a period of time (typically several weeks) under conditions such that analytical imprecision is minimized. The specimens should be handled identically and analysed in duplicate, using an identical technique (e.g. the same instrument, operator, calibrators and reagents). This may either be done at the time the specimens are collected or in a single batch. If the latter approach is adopted, the specimens must be stored in such a way that degradation of the analyte is prevented (e.g. by freezing at a sufficiently low temperature). Using a single batch procedure is preferable as it eliminates between-run analytical variation. If the specimens are analysed in a single batch, the analytical imprecision (see below) can be calculated from the differences in the duplicate analyses and is given by: where SD is the standard deviation and n is the number of pairs of data. If, however, specimens are analysed at the time they are taken, the analytical imprecision must be calculated from replicate analyses of quality control samples. The standard deviation of a single set of data from each individual is then calculated after excluding any outliers (‘fliers’) using an appropriate statistical test. This standard deviation will encompass both analytical variation and the intraindividual variation (SDI), such that: Since SDA is known, the intraindividual variation can then be calculated. It is also possible to calculate the interindividual variation (SDG, due to the difference in the individual homoeostatic set points for the analyte between each member of the group) by calculating the SD for all single sets of data for all subjects in the group, since this SD is given by: The calculations required can be performed using a nested analysis of variance technique as provided in many statistical software packages. An alternative approach is to calculate coefficients of variation (CV) (given by (SD × 100)/mean). Values for CV can be substituted for SD in these formulae because the means are similar. Some typical values of biological variation for frequently measured analytes are given in Table 2.4. (A reference to a more extensive list is provided in the Further reading section at the end of the chapter.) It should be noted that, while in most instances the interindividual variation is greater than the intraindividual variation, this is not always the case. When it is not, it means that the extent of natural variation around individuals’ homoeostatic set points is more than the range of variation between these set points. The relative sizes of intra- and interindividual variation have important consequences for the interpretation of analytical data in relation to reference ranges, as will be discussed later in the chapter. TABLE 2.4 Biological and analytical variation and consequent desirable analytical performance parameters for some frequently measured constituents of plasma expressed as coefficients of variation (CV = (SD × 100)/mean) Data from various sources, including UKNEQAS (UK National External Quality Assurance Scheme) website and Ricos et al. on Westgard QC website (see Further reading).
Acquisition and interpretation of biochemical data
INTRODUCTION
THE TEST REQUEST
Information
Reason
Patient’s name, identifying (e.g. hospital) numbera, date of birth and sex
Identification and (age, sex) interpretation of results
Home address for primary care patients
Address may be useful if grossly abnormal results are found
Return address (e.g. ward, clinic, surgery; telephone/pager number if urgent)
Delivery of report
Name of clinician (and telephone/pager number)
Liaison
Audit
Billing
Clinical details (including drug treatment)
Justification of request
Audit
Interpretation
Selection of appropriate assays
Choice of analytical method (to avoid drug interference)
Test(s) requested
Instruction to analyst
Sample(s) required
Instruction to phlebotomist
Date (and timeb if appropriate)
Identification
Interpretation (with timed/sequential requests)
FACTORS AFFECTING TEST RESULTS
Preanalytical factors
Technical factors
Biological factors
Factor
Example
Endogenous
Age
Cholesterol
Alkaline phosphatase
Urate
Sex
Gonadotrophins
Gonadal steroids
Body mass
Triglycerides
Exogenous
Time
Cortisol (daily)
Gonadotrophins (in women, catamenial)
25-Hydroxyvitamin D (seasonal)
Stress
Cortisol
Prolactin
Catecholamines
Posture
Renin
Aldosterone
Proteins
Food intake
Glucose
Triglycerides
Drugs
see Table 2.3 and text
Endogenous factors
Age
Sex
Ethnic origin
Body mass
Exogenous factors
Drugs
Effect
Mechanism
Glucocorticoids
Decreased plasma cortisol
Suppression of ACTH secretion
Thiazide diuretics
Decreased plasma potassium
Increased renal potassium excretion
Oestrogens
Increased plasma total thyroxine
Increased synthesis of binding proteins
Phenytoin, phenobarbitone, alcohol
Increased plasma γ-glutamyltransferase
Increased enzyme synthesis (enzyme induction)
Time-dependent changes
Stress
Posture
Food intake
Drugs
Other factors
Intrinsic biological variation
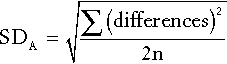
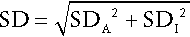
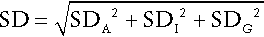
Analytical factors
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


