OUTLINE
When Is It Not Necessary to Add a Preservative?
When Are Preservatives Contraindicated?
Alternative Strategies When Preservatives Are Needed But Are Contraindicated
Properties of the Ideal Preservative
Antimicrobial Preservatives Listed in the USP 30–NF 25
Preservatives for Oral Dosage Forms
Preservatives for Topical Preparations
Preservatives for Ophthalmic Preparations
Need for Preservative-free Formulations
I. DEFINITION
Antimicrobial preservatives are substances added to nonsterile dosage forms to protect them from microbiological growth or from microorganisms that are introduced inadvertently during or subsequent to the manufacturing process. In the case of sterile articles packaged in multiple-dose containers, antimicrobial preservatives are added to inhibit the growth of microorganisms that may be introduced from repeatedly withdrawing individual doses.
Antimicrobial agents should not be used as a substitute for good manufacturing practices or solely to reduce the viable microbial population of a nonsterile product or to control the presterilization bioburden of multidose formulations during manufacturing (1). —USP
II. USES OF PRESERVATIVES
A. Preservatives should be added to extemporaneously compounded preparations when there is a possibility of microbial contamination and growth, either at the time of preparations or during use by the patient or caregiver (1,2).
B. In USP Chapter 〈1151〉 Pharmaceutical Dosage Forms, antimicrobial agents are explicitly mentioned and required for most dosage forms containing water and for one nonaqueous system, ophthalmic ointments (3).
1. Emulsions (including semisolid or ointment-type emulsions):“All emulsions require an antimicrobial agent because the aqueous phase is favorable to the growth of microorganisms.”
2. Suspensions: “Suspensions intended for any route of administration should contain suitable antimicrobial agents to protect against bacteria, yeast, and mold contamination.”
3. Oral solutions: “Antimicrobial agents to prevent the growth of bacteria, yeasts, and molds are generally also present.”
4. Ophthalmic solutions: “Each solution must contain a suitable substance or mixture of substances to prevent the growth of, or to destroy, microorganisms accidentally introduced when the container is opened during use. Where intended for use in surgical procedures, ophthalmic solutions, although they must be sterile, should not contain antibacterial agents, since they may be irritating to the ocular tissues.”
5. Ophthalmic ointments: “Ophthalmic ointments must contain a suitable substance or mixture of substances to prevent growth of, or to destroy, microorganisms accidentally introduced when the container is opened during use, unless otherwise directed in the individual monograph, or unless the formula itself is bacteriostatic.”
C. The requirements for antimicrobial agents in parenteral products are treated in USP Chapter 〈1〉, Injections (4):
A suitable substance or mixture of substances to prevent the growth of microorganisms must be added to preparations intended for injection that are packaged in multiple-dose containers, regardless of the method of sterilization employed, unless one of the following conditions prevails: (1) there are different directions in the individual monograph; (2) the substance contains a radionuclide with a physical half-life of less than 24 hours; (3) the active ingredients are themselves antimicrobial. Such substances are used in concentrations that will prevent the growth of or kill microorganisms in the preparations for injection (4).
III. WHEN IS IT NOT NECESSARY TO ADD A PRESERVATIVE?
A. The preparation will be used immediately. This assumes that the preparation is made using appropriate techniques that avoid contamination while it is being made and administered.
B. No water is present. Generally, microorganisms require water for growth, so products that contain no water, such as tablets, powders, and hydrocarbon ointments, are not media for growth. Exceptions to this rule include ophthalmic ointments and nonaqueous injections when the USP specifically requires an antimicrobial agent.
C. The pH of the medium is either <3 or >9.
Note: Though this pH range for inhibition of growth holds true for most microorganisms, certain resistant molds have been shown to grow in media with pH <3 (5).
D. Ingredient(s) that have antimicrobial properties are already present in the formulation.
IV. WHEN ARE PRESERVATIVES CONTRAINDICATED?
A. Neonates
B. Ophthalmic solutions intended for use in eyes during eye surgery, with non-intact corneas, or for intraocular injection
C. Parenteral products with volumes >30 mL
V. ALTERNATIVE STRATEGIES WHEN PRESERVATIVES ARE NEEDED BUT ARE CONTRAINDICATED
A. Prepare a single dose and use immediately.
B. Prepare a limited quantity that will be used within a short time period, store under refrigeration, and label with a short expiration period.
VI. PROPERTIES OF THE IDEAL PRESERVATIVE
A. Effective at a low, nontoxic concentration against a wide variety of organisms
B. Chemically stable under normal conditions of use over a wide pH and temperature range
C. Soluble at the required concentration
D. Compatible with a wide variety of drugs and excipients
E. Free from objectionable odor, taste, color, or stinging
F. Nontoxic and nonsensitizing both internally and externally at the required concentration
G. Reasonable cost
H. Unreactive (does not adsorb, penetrate, or interact) with containers or closures
VII. ANTIMICROBIAL PRESERVATIVES LISTED IN THE USP 30–NF 25
A. Table 16.1 gives the articles listed as antimicrobial preservatives in the USP 30–NF 25 (6). Some chemicals, though not listed by the USP as preservatives, have antimicrobial properties and may be useful as preservatives in formulated preparations.
B. The preservatives used most commonly in extemporaneous compounding are described in sections VIII through X of this Chapter.
1. To facilitate selection of an antimicrobial preservative when formulating a preparation, the agents are organized by suitability for route of administration (e.g., oral, topical, and ophthalmic).
2. The descriptions and solubilities presented here give a composite of information from the Chemistry and Compendial Requirements section of the USP DI Vol. III (7), The Merck Index (8), the Handbook of Pharmaceutical Excipients (2), and other references as cited. For additional information on each agent, consult the Handbook of Pharmaceutical Excipients.
C. In reading and interpreting the current chapter, note that this text employs the usual convention of using upper-case first letters for words designating official USP-NF articles (e.g., Alcohol, Purified Water) and lower-case first letters for words designating the chemical substances (e.g., ethanol, water).
VIII. PRESERVATIVES FOR ORAL DOSAGE FORMS
A. Alcohols and glycols
1. Ethyl Alcohol

a. Official articles of ethyl alcohol that are suitable for oral use:
(1) Alcohol USP
(2) Dehydrated Alcohol USP
(3) Dehydrated Alcohol Injection USP
(4) Diluted Alcohol NF
Note: Rubbing Alcohol USP is a product containing ethyl alcohol, but it may not be used orally. It contains denaturants that are toxic orally.
b. Description and solubility: See Chapter 15, Pharmaceutical Solvents and Solubilizing Agents
c. Effective concentration
(1) Effective concentration depends on the pH of the solution and the amount of “free water.”
Note: “Free water” is the water in a preparation that is not bound by interaction with other molecules. Originally, the term was used to represent the amount of water in a preparation that is not tied up by interaction with sucrose in the ratio of 85 g of sucrose to 45 mL of water. This 85:45 ratio of sucrose to water is called the USP Syrup Equivalent because it is the proportion of these ingredients in the formula for Syrup NF (Simple Syrup), which is a saturated, self-preserving solution. Obviously, other dissolved molecules can also reduce the activity of water. For example, a salt, such as potassium chloride, or a highly hydrated polymer, such as methylcellulose, also binds water in an aqueous solution, but the degree of these interactions has not been documented. It is important to realize that these interactions do occur, and when these ingredients are in a liquid preparation, they do effectively reduce the “free water” in that product.
(2) The alcohol concentration should be 15% (Acid)—17.5% (Neutral or mildly alkaline) of the free water (9).
(3) Though the 15% to 17.5% range seems high, the percentage actually needed for most preparations is far less because these percentages apply only to the “free water.” Consider the following example:
Mineral Oil Emulsion USP (10)
Mineral Oil | 500 mL |
Acacia | 125 g |
Syrup | 100 mL |
Vanillin | 40 mg |
Alcohol | 60 mL |
Purified Water, a sufficient quantity to make | 1,000 mL |
If you add the approximate volumes of all the ingredients (in the case of the solids—acacia and vanillin—you have to estimate the powder volume) and subtract this quantity from 1,000 mL, you get the approximate free water volume: 1,000 mL − [500 + 100 + 60 + ~40 (powder volume estimate)] = 300 mL. This is the amount of water that must be preserved with alcohol. The quantity of ethyl alcohol in this prescription is 95% × 60 mL = 57 mL. This quantity represents only 5.7% of the total product, but it is 19% of the free water (19% × 300 mL = 57 mL).
Some syrups are protected from microbial growth by virtue of their high solute concentrations that create an unfavorable osmotic environment for these organisms. However, more dilute syrups are good media for microbial growth and require the addition of preservatives. This is another example in which alcohol or another preservative should be added to preserve the free water.
(4) One commonly seen guideline of alcohol content for preservation of liquid formulations is 5% to 10% alcohol. In using this figure, one should be aware of its origins: It comes from a rough estimate of the free water available in the “average” product. Therefore, if you have a prescription order that contains a large proportion of free water, you should add extra alcohol for adequate preservation.
2. Propylene Glycol
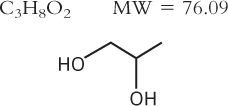
a. Official article Propylene Glycol USP
b. Description and solubility: See Chapter 15, Pharmaceutical Solvents and Solubilizing Agents.
c. Effective concentration
(1) In most situations, propylene glycol is effective at a concentration of 10% w/v, although inhibition of growth of certain molds requires up to 30% w/v (11,12).
(2) Propylene glycol potentiates several other preservatives. A 2% to 5% concentration of propylene glycol, though ineffective as a sole preservative, potentiates the effect of a methyl and propyl paraben combination. Various combinations tested were found to be effective against bacteria, molds, and yeast but were ineffective against bacterial spores until they entered the vegetative stage (13). This effect on paraben efficacy is especially useful because the concentration at which the parabens exert their antimicrobial effect is so close to their solubility. Though parabens are particularly useful for preserving vulnerable syrups that have a neutral pH, because of their poor water solubility, it is often difficult to dissolve a sufficient concentration of parabens to achieve adequate preservation. In this study, both a 2% and a 5% concentration of propylene glycol allowed the minimum inhibitory concentration of methylparaben to be reduced from 0.18% to 0.1% when combined with 0.02% propylparaben (13).
3. Glycerin
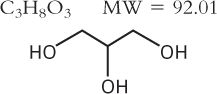
a. Official article Glycerin USP
b. Description and solubility: See Chapter 15, Pharmaceutical Solvents and Solubilizing Agents.
c. Effective concentration
(1) Though glycerin preserves at concentrations ≥50%, at lower concentrations it may actually act as a nutrient for some microorganisms. This is because glycerin’s activity as an antimicrobial agent depends solely on an osmotic effect rather than any innate toxicity to microorganisms (13).
(2) Though glycerin is not often used as a sole preservative, it is frequently used with alcohol to reduce the volume of alcohol necessary to preserve a preparation.
4. Benzyl Alcohol
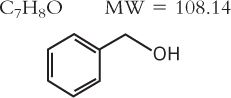
a. Official article Benzyl Alcohol NF
b. Description: colorless liquid; faint, aromatic odor; sharp, burning taste; neutral pH; flammable
c.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree