Medication Drug Orders
CHAPTER
OUTLINE
Issuing and Receiving Drug Orders
Outpatient Prescription Drug Orders
Records for Compounded Drug Orders
Recommended Practices to Prevent Medication Errors in Drug Orders
I. INTRODUCTION AND DEFINITIONS
A. The starting point for furnishing drug therapy to patients is the prescription or medication drug order. When discussing required and recommended elements of these drug orders and identifying both authorized personnel and safe and effective procedures for handling the various phases of the drug order process, it is essential to have some basic definitions.
B. The definitions given in this section are taken from the Model State Pharmacy Act of the National Association of Boards of Pharmacy. The National Association of Boards of Pharmacy (NABP) publishes a Model State Pharmacy Act and model rules for various areas of pharmacy practice. Although states are encouraged to use these in formulating individual state laws, each state is free to enact its own laws and administrative codes regulating the practices of pharmacy and medicine. To learn the specific definitions and legal requirements for the state where you are practicing, consult the applicable state statutes. The Model State Pharmacy Act and the Model Rules of the National Association of Boards of Pharmacy are revised and updated frequently; a current copy is available on their Internet Web site at http://www.nabp.net, Accessed December 2007.
C. Definitions: Because the language for these definitions has been carefully chosen by the NABP, most definitions given here are direct quotes from the 2007 Model Act. (Note that the Act uses uppercase beginning letters for all words or terms that are given specific definitions in the Act.)
1. The Practice of Pharmacy is defined in Section 104 of the NABP Model Act as follows:
The “Practice of Pharmacy” means the interpretation, evaluation, and implementation of Medical Orders; the Dispensing of Prescription Drug Orders; participation in Drug and Device selection; Drug Administration; Drug Regimen Review; the Practice of Telepharmacy within and across state lines; Drug or Drug-related research; the provision of Patient Counseling; the provision of those acts or services necessary to provide Pharmacist Care in all areas of patient care, including Primary Care and Collaborative Pharmacy Practice; and the responsibility for Compounding and Labeling Drugs and Devices (except Labeling by a Manufacturer, Repackager, or Distributor of Non-Prescription Drugs and commercially packaged Legend Drugs and Devices), proper and safe storage of Drugs and Devices, and maintenance of required records. (1)
2. Dispensing: “The interpretation, evaluation, and implementation of a Prescription Drug Order, including the preparation and Delivery of a Drug or Device to a patient or patient’s agent in a suitable container appropriately labeled for subsequent Administration to, or use by, a patient” (2).
3. Pharmacist: “An individual currently licensed by this State to engage in the Practice of Pharmacy” (3).
4. Pharmacy Technician: “Personnel registered with the Board [of Pharmacy] who may, under the supervision of the Pharmacist, assist in the pharmacy and perform such functions as assisting in the Dispensing process; processing of medical coverage claims; stocking of medications; cashiering but excluding Drug Regimen Review; clinical conflict resolution; prescriber contact concerning Prescription Drug Order clarification or therapy modification; Patient Counseling; Dispensing process validation; prescription transfer; and receipt of new Prescription Drug Orders” (4). It is important to note that not all states have registration of pharmacy technicians, and individual states differ with respect to the scope of allowed activities for pharmacy technicians and the required level of supervision by the pharmacist. Therefore, it is essential that you consult state statutes and/or applicable regulations for your practice site.
5. Certified Pharmacy Technician: “Personnel registered with the Board [of Pharmacy] who have completed a certification program approved by the Board and may, under supervision of a Pharmacist, perform certain activities involved in the Practice of Pharmacy, such as receiving new Prescription Drug Orders; prescription transfer; and Compounding but excluding Drug Regimen Review; clinical conflict resolution; prescriber contact concerning Prescription Drug Order clarification or therapy modification; Patient Counseling; and Dispensing process validation.” (5) As is the case for pharmacy technicians, not all states have registration of certified pharmacy technicians; furthermore, some states that register such personnel do not make a legal distinction, including allowed activities, between pharmacy technicians and certified pharmacy technicians. Because states differ with respect to the scope of allowed activities of certified pharmacy technicians, it is essential to obtain this information by consulting the state statutes or applicable regulations where you are practicing.
6. Practitioner: “An individual currently licensed, registered, or otherwise authorized by the appropriate jurisdiction to prescribe and Administer Drugs in the course of professional practice” (4). In most cases the “appropriate jurisdiction” is the state, but the federal government has such authority for federal facilities such as military bases and veterans’ hospitals and clinics. Traditionally practitioners have included medical and osteopathic doctors, dentists, and veterinarians, but now others such as podiatrists, physician assistants, and nurse practitioners may also be given authority to prescribe and administer drugs. For more specifics on this topic, see the information on prescribing authority in section II of this chapter.
7. Prescription Drug or Legend Drug: “A Drug which is required by any applicable Federal or State law or rule to be Dispensed pursuant only to a Prescription Drug Order or is restricted to use by Practitioners only” (4). The term “Legend Drug” comes from the legend that is required on the manufacturer or distributor’s label on containers of such drugs. The legend states “Caution: Federal law prohibits dispensing without a prescription,” “Caution: Federal law restricts this drug to use by, or on the order of, a licensed veterinarian,” “Rx only,” or a similar phrase.
8. Prescription Drug Order: “A lawful order from a Practitioner for a Drug or Device for a specific patient, including orders derived from Collaborative Pharmacy Practice, that is communicated to a Pharmacist in a licensed Pharmacy” (4). The terms “prescription drug order,” “prescription order” and “prescription” are used interchangeably by health care workers and the public. The slang terms “Rx” and “script” are also sometimes used by pharmacists, technicians, and other health care workers. These various terms are most commonly used to describe drug orders for ambulatory patients (also referred to as “outpatients”) who get their prescribed medications from retail or clinic pharmacies. The terms “medication order” and “chart drug order” are often used when referring to drug orders for persons who are patients in hospitals, nursing homes, or other institutional settings. These patients are referred to as “inpatients.”
9. Medical Order: “A lawful order of a Practitioner that may or may not include a Prescription Drug Order” (6).
10. Chart Order: “A lawful order entered on the chart or a medical record of an inpatient or resident of an Institutional Facility by a Practitioner or his or her designated agent for a Drug or Device” (7). A chart order is considered a prescription drug order if it contains the usual elements of a prescription drug order, such as the name of the patient, date of the order, name, strength, and dosage form of the prescribed drug, directions for use, and name or signature of the prescriber (7).
11. Compounding: “The preparation of Components into a Drug product (1) as the result of a Practitioner’s Prescription Drug Order or initiative based on the Practitioner/patient/Pharmacist relationship in the course of professional practice, or (2) for the purpose of, or as an incident to, research, teaching, or chemical analysis and not for sale or Dispensing. Compounding also includes the preparation of Drugs or Devices in anticipation of receiving Prescription Drug Orders based on routine, regularly observed prescribing patterns” (7).
12. Collaborative Pharmacy Practice Agreement: A written and signed agreement between a pharmacist and licensed practitioner that allows the pharmacist to initiate or modify a patient’s drug regimen within the guidelines of an agreed protocol for the purpose of drug therapy management (7,8). Collaborative Pharmacy Practice Agreements are often used by pharmacists and physicians or other practitioners to manage patients with specific disease states such as diabetes or asthma or intense drug therapy such as anticoagulation.
II. ISSUING AND RECEIVING DRUG ORDERS
A. Prescribing authority
1. As described earlier in the definition of “practitioner”, state statutes regulate which licensed health care providers may prescribe and administer drugs in that state. In federal facilities, such as military or veterans’ hospitals and clinics, the federal government stipulates who has this authority.
2. Medical and osteopathic doctors, podiatrists, dentists, and veterinarians have traditionally been the practitioners given authority to prescribe drugs; however, many states now also give this authority to optometrists, nurse practitioners, physician assistants, psychologists, and/or pharmacists. In the later cases, certain restrictions may apply. For example, such practitioners may be required to possess advanced degrees or to pass special certification exams, or they may be restricted to prescribing only under the supervision of a licensed physician or under a limited, established protocol, such as a collaborative pharmacy practice agreement. It is the duty of pharmacists to stay informed about laws regulating current prescribing authority for health professionals in their practice area.
3. In all cases, practitioners are restricted to prescribing within the scope of their practice and for a legitimate medical purpose (9). For example, veterinarians can prescribe only for animals; dentists are limited to prescribing medications required by their dental patients for their dental problems, and so on.
4. An institutional facility, such as a hospital or nursing home, may further restrict who may prescribe for patients in that facility.
5. If allowed by the appropriate jurisdiction, practitioners may delegate to a designated agent certain portions of the physical act of writing or transmitting the prescription order.
B. Transmitting and receiving prescription drug orders
1. Pharmacists are the individuals authorized to receive prescription drug orders. In some states, certified pharmacy technicians are allowed to perform this function, provided there is a system in place that allows the pharmacist to review the prescription drug order as transmitted to the technician (9).
2. There are several methods allowed for transmitting prescription drug orders from practitioner to pharmacist:
a. Written prescription drug orders may be used for any legal drugs, including controlled substances in Schedules II through V (9,10). See Chapter 3 of this book for additional information on prescription drug order regulations for controlled substances.
b. In most states, orders by electronic transmission, facsimile or fax, and e-prescribing are acceptable for legal drugs and for controlled substances in Schedules III through V. Under certain prescribed circumstances, these methods may also be used for controlled substances in Schedule II (9,10). See applicable state statutes and federal laws for your practice area for regulations on transmitting prescription orders electronically.
c. Verbal orders, including both face-to-face and telephone voice communication, are allowed for legal drugs and controlled substances in Schedules III through V. As with electronic orders, under certain prescribed circumstances, verbal orders may also be used for controlled substances in Schedule II (9,10). See applicable state statutes, federal laws, and Chapter 3 of this book for additional information on these regulations for controlled substances.
(1) Traditionally it was the rule that verbal orders be “reduced to writing” immediately after receiving the order. With electronic record-keeping systems, this rule has been broadened; the NABP Model Rules for the Practice of Pharmacy now state that orders transmitted verbally or electronically “shall be immediately reduced to a form [emphasis added] by the Pharmacist or Certified Pharmacy Technician that may be maintained for the time required by laws or rules” (9).
(2) Because verbal orders may easily be misunderstood, they are one source of medication errors. For this reason, current practice standards discourage the use of verbal orders except in urgent situations (11,12). Recent standards state that the person receiving the order record and “read back” [emphasis added] the complete order (11–13).
d. Both written and verbal orders have been one source of medication errors. For this reason, various health care organizations have been working to establish for this area of practice standards that will minimize errors and ensure greater safety for patients. These standards and recommendations are discussed in section VI of this chapter.
III. OUTPATIENT PRESCRIPTION DRUG ORDERS
A. In addition to regulating who may prescribe and who may receive and process prescription drug orders, state statutes also spell out what information is required on these drug orders and what must be kept in records of dispensing.
B. Required information on prescription drug orders

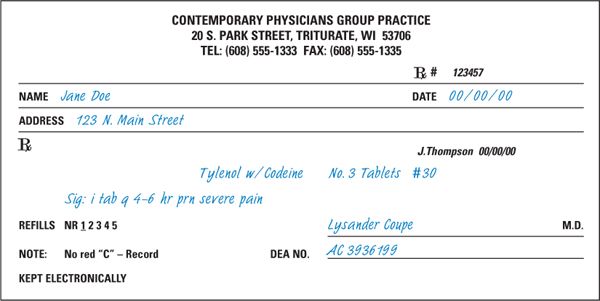
1. The information given in this section concerning recommended legal requirements for prescription drug orders comes from that section of the Model State Pharmacy Act of the National Association of Boards of Pharmacy entitled Model Rules for the Practice of Pharmacy (9). The NABP recommended elements are outlined below and are illustrated in Figures 1.1 and 1.2 and on the CD that accompanies this book. To learn the specific legal requirements for the state where you are practicing, consult the applicable state statutes. For the samples in Figures 1.1 and 1.2, the information printed in blue simulated handwriting type denotes those items that are normally entered on the prescription order by the prescriber. Those elements on the samples that are printed in black simulated handwriting are added by the pharmacist at the time of dispensing and are described in section D.
a. Full name and address of the patient
Note: If the patient is an animal, the full name is generally considered to include the animal’s name, species, and owner.
FIGURE 1.1. PRESCRIPTION ORDER FOR A GENERIC DRUG PRODUCT.
FIGURE 1.2. PRESCRIPTION ORDER FOR A BRANDED, CONTROLLED SUBSTANCE DRUG PRODUCT.
b. Date of issue
c. Name and address of the prescriber
d. Name, strength, dosage form, and quantity of the drug product prescribed
e. Directions for use
f. Refills authorized, if any
Note:Accepted interpretation of refill, or lack of refill, information may be specified in state law. Common interpretations include the following:
(1) For drug products available only on prescription, the absence of refill information usually means zero refills are authorized.
(2) The number of allowed refills for controlled substances is specified in both state and federal law. Information on this topic can be found in Chapter 3 of this book and in the applicable laws.
(3) The designation “prn” refills for prescription medications that are not controlled substances is limited to authorizing refills for 1 year, or other specified time period, from the date of issue.
g. Prescriber’s signature (written orders)
h. For controlled substances, the following additional requirements apply:
(1) DEA registration number of the prescriber
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree