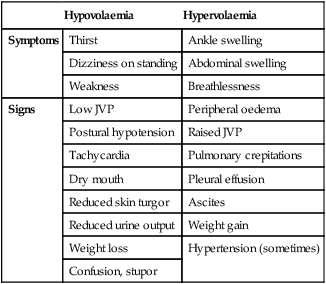
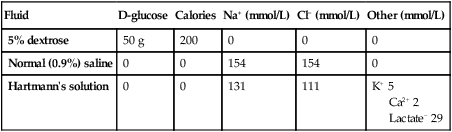
16 There are three broad reasons why a clinician may request a biochemical laboratory investigation: • to screen an asymptomatic subject for the presence of disease • to assist in diagnosis of a patient’s presenting complaint • to monitor changes in test results, as a marker of disease progression or response to treatment. • Large central biochemistry laboratories feature extensive use of automation and information technology. Specimens are transported from clinical areas to the laboratory using high-speed transport systems (such as pneumatic tubes) and identified with machine-readable labels (such as bar codes). Laboratory instruments have been miniaturised and integrated with robot transport systems to enable multiple rapid analyses of a single sample. Statistical process control techniques are used to assure the quality of analytical results, and increasingly to monitor other aspects of the laboratory, such as the time taken to complete the analysis (‘turn-around time’). • Point-of-care testing (POCT) brings selected laboratory analytical systems into clinical areas, to the patient’s bedside or even connected to an individual patient. These systems allow the clinician to receive results almost instantaneously for immediate treatment of the patient, although often with less precision or at greater cost than using a central laboratory. • The diversity of analyses has widened considerably with the introduction of many techniques borrowed from the chemical or other industries (Box 16.1). Good medical practice involves the appropriate ordering of laboratory investigations and correct interpretation of test results. The key principles, including the concepts of sensitivity and specificity, are described on page 5. Reference ranges for laboratory results are provided in Chapter 29. Many laboratory investigations can be subject to variability arising from incorrect patient preparation (for example, in the fasting or fed state), timing of sample collection (for example, in relation to diurnal variation of hormone levels, or dosage regimens for therapeutic agents), analytical factors (for example, serum versus plasma; use of the correct anticoagulant, or POCT versus central analysis) or artefact (for example, taking a venous sample proximal to the site of an intravenous infusion). It is therefore important for clinical and laboratory staff to communicate effectively and for clinicians to follow local recommendations concerning collection and transport of samples in the appropriate container and with appropriate labelling. One of the most common uses of the clinical biochemistry laboratory is to monitor electrolyte and acid–base status. The diverse clinical consequences of these biochemical disorders are illustrated in Box 16.2. Some whole-body electrolyte disturbances (notably of sodium) result in major clinical problems with minimal disturbance in measured biochemical parameters. However, these will also be considered for convenience here. In a typical adult male, total body water (TBW) is approximately 60% of body weight (somewhat more for infants and less for women). For an average individual, TBW is about 40 L. Approximately 25 L is located inside cells (the intracellular fluid or ICF), while the remaining 15 L is in the extracellular fluid (ECF) compartment (Fig. 16.1). Most of the ECF (approximately 12 L) is interstitial fluid, which is within the tissues but outside cells, whereas the remainder (about 3 L) is in the plasma compartment. Figure 16.1 illustrates some of the major differences in composition between the main body fluid compartments. The dominant cation in the ICF is potassium, while the dominant cation in the ECF is sodium. Phosphates and negatively charged proteins constitute the major intracellular anions, while chloride and, to a lesser extent, bicarbonate dominate the ECF anions. An important difference between the plasma and interstitial compartments of the ECF is that only plasma contains significant concentrations of protein. The most common biochemical test in plasma is called the urea and electrolytes (U&E) test in some parts of the world, and the electrolytes/urea/creatinine (EUC) in others. A guide to its interpretation is shown in Box 16.3. Because the blood consists of both intracellular (red cell) and extracellular (plasma) components, it is important to avoid haemolysis during or after collection of the sample, which causes contamination of the plasma compartment by intracellular elements, particularly potassium. Blood should not be drawn from an arm into which an intravenous infusion is being given, to avoid contamination by the infused fluid. Repeated measurements of plasma electrolytes are frequently necessary when abnormalities have been detected and corrective therapy instituted. Since the kidney maintains the constancy of body fluids by adjusting urine volume and composition, it is frequently helpful to obtain a sample of urine (‘spot’ specimen or 24-hour collection) at the time of blood analysis. An example of the use of urine biochemistry is given for the differential diagnosis of hyponatraemia in Box 16.14 (p. 438). The functional unit for renal excretion is the nephron (Fig. 16.2). Blood undergoes ultrafiltration in the glomerulus, generating a fluid that is free from cells and protein and which resembles plasma in its electrolyte composition. This is then delivered into the renal tubules, where reabsorption of water and various electrolytes occurs (more detail on the structure and function of the glomerulus is given in Ch. 17). The glomerular filtration rate (GFR) is approximately 125 mL/min (equivalent to 180 L/day) in a typical adult. Over 99% of this filtered fluid is reabsorbed into the blood in the peritubular capillaries during its passage through successive segments of the nephron, largely as a result of tubular reabsorption of sodium. The processes mediating this sodium reabsorption, and the factors that regulate it, are key to understanding clinical disturbances and pharmacological interventions. At least four different functional segments of the nephron can be defined in terms of their mechanism for sodium reabsorption (Fig. 16.3). This is responsible for the reabsorption of some 65% of the filtered sodium load. The cellular mechanisms are complex but some of the key features are shown in Figure 16.3A. Filtered sodium in the luminal fluid enters the cell via several sodium transporters in the apical membrane that couple sodium transport to the entry of glucose, amino acid, phosphate and other organic molecules. Entry of sodium into the tubular cells at this site is also linked to secretion of H+ ions, through the sodium–hydrogen exchanger (NHE-3). Intracellular H+ ions are generated within tubular cells from the breakdown of carbonic acid, which is produced from carbon dioxide and water under the influence of carbonic anhydrase. Large numbers of Na,K-ATPase pumps are present on the basolateral membrane of tubular cells that transport sodium from the cells into the blood. In addition, a large component of the transepithelial flux of sodium, water and other dissolved solutes occurs through the gaps between the cells (the ‘shunt’ pathway). Overall, fluid and electrolyte reabsorption is almost isotonic in this segment, as water reabsorption is matched very closely to sodium fluxes. A component of this water flow also passes through the cells, via aquaporin-1 (AQP-1) water channels, which are not sensitive to hormonal regulation. The thick ascending limb of the loop of Henle (Fig. 16.3B) reabsorbs a further 25% of the filtered sodium but is impermeable to water, resulting in dilution of the luminal fluid. Again, the primary driving force for sodium reabsorption is the Na,K-ATPase on the basolateral cell membrane, but in this segment sodium enters the cell from the lumen via a specific carrier molecule, the Na,K,2Cl co-transporter (‘triple co-transporter’, or NKCC2), which allows electroneutral entry of these ions into the renal tubular cell. Some of the potassium accumulated inside the cell recirculates across the apical membrane back into the lumen through a specific potassium channel (ROMK), providing a continuing supply of potassium to match the high concentrations of sodium and chloride available in the lumen. A small positive transepithelial potential difference exists in the lumen of this segment relative to the interstitium, and this serves to drive cations such as sodium, potassium, calcium and magnesium between the cells, forming a reabsorptive shunt pathway. Some 6% of filtered sodium is reabsorbed in the early distal tubule (also called distal convoluted tubule) (Fig. 16.3C), again driven by the activity of the basolateral Na,K-ATPase. In this segment, entry of sodium into the cell from the luminal fluid is via a sodium–chloride co-transport carrier (NCCT). This segment is also impermeable to water, resulting in further dilution of the luminal fluid. There is no significant transepithelial flux of potassium in this segment, but calcium is reabsorbed through the mechanism shown in Figure 16.3C: a basolateral sodium–calcium exchanger leads to low intracellular concentrations of calcium, promoting calcium entry from the luminal fluid through a calcium channel. The late distal tubule and cortical collecting duct are anatomically and functionally continuous (Fig. 16.3D). Here, sodium entry from the luminal fluid occurs via the epithelial sodium channel (ENaC) through which sodium passes alone, generating a substantial lumen-negative transepithelial potential difference. This sodium flux into the tubular cells is balanced by secretion of potassium and hydrogen ions into the lumen and by reabsorption of chloride ions. Potassium is accumulated in the cell by the basolateral Na,K-ATPase, and passes into the luminal fluid down its electrochemical gradient, through an apical potassium channel (ROMK). Chloride ions pass largely between cells. Hydrogen ion secretion is mediated by an H+-ATPase located on the luminal membrane of the intercalated cells, which constitute approximately one-third of the epithelial cells in this nephron segment. This part of the nephron has a variable permeability to water, depending on the availability of antidiuretic hormone (ADH, or vasopressin) in the circulation. All ion transport processes in this segment are stimulated by the steroid hormone aldosterone, which can increase sodium reabsorption in this segment to a maximum of 2–3% of the filtered sodium load. A large number of interrelated mechanisms serve to maintain whole body sodium balance and hence ECF volume by matching urinary sodium excretion to sodium intake (Fig. 16.4). • reduced perfusion pressure in the afferent arteriole • increased sympathetic nerve activity • decreased sodium chloride concentration in the distal tubular fluid. Renin released into the circulation activates the effector mechanisms for sodium retention, which are components of the renin–angiotensin–aldosterone (RAA) system (see Fig. 20.17, p. 771). Renin acts on the peptide substrate, angiotensinogen (manufactured in the liver), producing angiotensin I in the circulation. This in turn is cleaved by angiotensin-converting enzyme (ACE) into angiotensin II, largely in the pulmonary capillary bed. Angiotensin II has multiple actions: it stimulates proximal tubular sodium reabsorption and release of aldosterone from the zona glomerulosa of the adrenal cortex, and causes vasoconstriction of small arterioles. Aldosterone amplifies sodium retention by its action on the cortical collecting duct. The net effect is to restore ECF volume and blood pressure towards normal, thereby correcting the initiating hypovolaemic stimulus. When the balance of sodium intake and excretion is disturbed, any tendency for plasma sodium concentration to change is usually corrected by the osmotic mechanisms controlling water balance (p. 436). As a result, disorders in sodium balance present chiefly as alterations in the ECF volume, resulting in hypovolaemia or oedema, rather than as an alteration in plasma sodium concentration. Clinical manifestations of altered volume are illustrated in Box 16.4. Sodium depletion can occur occasionally under extreme environmental conditions due to inadequate intake of salt, but it is much more commonly due to pathological losses of sodium-containing fluids (Box 16.5). Loss of whole blood, as in acute haemorrhage, is also an obvious cause of hypovolaemia, and elicits the same mechanisms for the conservation of sodium and water. The diagnosis of hypovolaemia is based on characteristic symptoms and signs (see Box 16.4) in the context of a relevant precipitating illness. Supportive evidence may be obtained from the clinical biochemistry laboratory. Although plasma sodium concentration may not be reduced if salt and water are lost in equal proportions, a number of other parameters are altered during appropriate renal, hormonal and haemodynamic responses to hypovolaemia. During the early stages of hypovolaemia, GFR is maintained while urinary flow rate is reduced as a consequence of activation of sodium- and water-retaining mechanisms in the nephron. Thus, plasma creatinine, which reflects GFR, may be relatively normal, but the plasma urea concentration is typically elevated, since urea excretion is affected by both GFR and urine flow rate. Plasma uric acid may also rise, reflecting activation of compensatory proximal tubular reabsorption. With avid retention of sodium and water, the urine osmolality increases while the urine sodium concentration falls. Under these circumstances, sodium excretion may fall to less than 0.1% of the filtered sodium load. Management of sodium and water depletion has two main components: • treat the cause where possible, to stop ongoing salt and water losses • replace the salt and water deficits, and provide ongoing maintenance requirements, usually by intravenous fluid replacement when depletion is severe. Box 16.6 shows the daily maintenance requirements for water and electrolytes in a typical adult, and Box 16.7 summarises the composition of some widely available intravenous fluids. The choice of fluid and the rate of administration depend on the clinical circumstances, as assessed at the bedside and from laboratory data, and as described in Box 16.8. In the absence of normal oral intake (as in a fasting or post-operative patient in hospital), maintenance quantities of fluid, sodium and potassium should be provided. If any deficits or continuing pathological losses are identified, additional fluid and electrolytes will be required. In prolonged periods of fasting (more than a few days), attention also needs to be given to providing sufficient caloric and nutritional intake to prevent excessive catabolism of body energy stores (p. 120). The choice of intravenous fluid therapy in the treatment of significant hypovolaemia relates to the concepts in Figure 16.1 (p. 429). If fluid containing neither sodium nor protein is given, it will distribute in the body fluid compartments in proportion to the normal distribution of total body water. Thus, giving 1 L of 5% dextrose will contribute relatively little (approximately 3/40 of the infused volume) towards expansion of the plasma volume. This makes 5% dextrose ineffective at restoring the circulation and perfusion of vital organs. Intravenous infusion of an isotonic (normal) saline solution, on the other hand, results in more effective expansion of the extracellular fluid, although a minority of the infused volume (some 3/15) will contribute to plasma volume. Carrying this reasoning further, it might be expected that a solution containing plasma proteins would be largely retained within the plasma, thus maximally expanding the circulating fluid volume and improving tissue perfusion. However, recent clinical studies have not shown any overall advantage of infusions containing albumin in the treatment of acute hypovolaemia (Box 16.9). Resuscitation fluids containing synthetic colloids such as carbohydrate polymers should not be used in the acute resuscitation of volume-depleted patients since they offer no benefit over crystalloids and are associated with increased mortality (see Box 17.21, p. 482). In patients with normal cardiac and renal function, excessive intakes of salt and water are compensated for by increased excretion and do not lead to clinically obvious features of sodium and water overload. However, patients with cardiac, renal or hepatic disease frequently present with signs and symptoms of sodium excess (Fig. 16.5). This does not always involve an increase in circulating blood volume, since the excess fluid often leaks out of the capillaries to expand the interstitial compartment of the ECF, especially in diseases like nephrotic syndrome and chronic liver disease that cause hypoalbuminaemia. Important causes of sodium excess are shown in Box 16.10. Peripheral oedema is the most common physical sign of ECF volume expansion (p. 478). The three most common systemic disorders associated with sodium and fluid overload are cardiac failure, cirrhosis and nephrotic syndrome. In each of these, sodium retention is largely a secondary response to circulatory insufficiency caused by the primary disorder, as illustrated in Figure 16.5. The pathophysiology is different in renal failure, when the primary cause of volume expansion is the profound reduction in GFR impairing sodium and water excretion, and secondary tubular mechanisms are of lesser importance. Further detail on each of these conditions is given in other chapters of this book. Diuretics are important in the treatment of conditions of ECF expansion due to salt and water retention and in hypertension (p. 606). They act by inhibiting sodium reabsorption at various locations along the nephron (see Fig. 16.3, p. 431). Their potency and adverse effects relate to their mechanism and site of action. All diuretic drugs acting in the proximal, loop and early distal segments cause excretion not only of sodium (and with it water), but also of potassium. This occurs largely as a result of delivery of increased amounts of sodium to the late distal/cortical collecting ducts, where sodium reabsorption is associated with excretion of potassium, and is amplified if circulating aldosterone levels are high. By contrast, drugs acting to inhibit sodium reabsorption in the late distal/cortical collecting duct segment are associated with reduced potassium secretion, and are described as ‘potassium-sparing’. One target of drug action in this segment is the apical sodium channel in the principal cells (see Fig. 16.3), which is blocked by drugs such as amiloride and triamterene. Another is the mineralocorticoid receptor, to which binding of aldosterone is blocked by spironolactone and eplerenone. Adverse effects encountered with the most commonly used classes of diuretic (loop drugs and thiazide drugs) are summarised in Box 16.11. Volume depletion and electrolyte disorders commonly occur, as predicted from their mechanism of action. The metabolic side-effects listed are rarely of clinical significance and may reflect effects on K+ channels that influence insulin secretion (p. 800). Since most drugs from these classes are sulphonamides, there is a relatively high incidence of hypersensitivity reactions, and occasional idiosyncratic side-effects in a variety of organ systems. These functions are largely achieved by the loop of Henle and the collecting ducts. The counter-current configuration of flow in adjacent limbs of the loop (see Fig. 16.2, p. 430), involves osmotic movement of water from the descending limbs and reabsorption of solute from neighbouring ascending limbs, to set up a gradient of osmolality from isotonic (like plasma) in the renal cortex to hypertonic (around 1200 mmol/kg) in the inner part of the medulla. At the same time, the fluid emerging from the thick ascending limb is hypotonic compared to plasma, because it has been diluted by the reabsorption of sodium, but not water, from the thick ascending limb and early distal tubule. As this dilute fluid passes from the cortex through the collecting duct system to the renal pelvis, it traverses the medullary interstitial gradient of osmolality set up by the operation of the loop of Henle, and water is reabsorbed. Further changes in the urine osmolality on passage through the collecting ducts depend on the circulating level of antidiuretic hormone (ADH), which is released by the posterior pituitary gland under conditions of increased plasma osmolality or hypovolaemia (Ch. 20). • When water intake is high and plasma osmolality is normal or low-normal, ADH levels are suppressed and the collecting ducts remain impermeable to water. The luminal fluid osmolality remains low, resulting in the excretion of a dilute urine (minimum osmolality approximately 50 mmol/kg in a healthy young person). • When water intake is restricted and plasma osmolality is high, or in the presence of plasma volume depletion, ADH levels rise. This causes water permeability of the collecting ducts to increase through binding of ADH to the V2 receptor, which enhances collecting duct water permeability through the insertion of aquaporin (AQP-2) channels into the luminal cell membrane. This results in osmotic reabsorption of water along the entire length of the collecting duct, with maximum urine osmolality approaching that in the medullary tip (up to 1200 mmol/kg). In summary, for adequate dilution of the urine there must be: • adequate solute delivery to the loop of Henle and early distal tubule • normal function of the loop of Henle and early distal tubule If any of these processes is faulty, water retention and hyponatraemia may result. Conversely, to achieve concentration of the urine there must be: • adequate solute delivery to the loop of Henle • normal function of the loop of Henle • ADH release into the circulation Failure of any of these steps may result in inappropriate water loss and hypernatraemia. Hyponatraemia (plasma Na < 135 mmol/L) is a common electrolyte abnormality, which is often asymptomatic but which can also be associated with profound disturbances of cerebral function, manifesting as anorexia, nausea, vomiting, confusion, lethargy, seizures and coma. The likelihood of symptoms occurring is related more to the speed at which electrolyte abnormalities develop rather than their severity. When plasma osmolality falls rapidly, water flows into cerebral cells, which become swollen and ischaemic. However, when hyponatraemia develops gradually, cerebral neurons have time to respond by reducing intracellular osmolality, through excreting potassium and reducing synthesis of intracellular organic osmolytes (Fig. 16.6). The osmotic gradient favouring water movement into the cells is thus reduced and symptoms are avoided. The causes of hyponatraemia are best categorised according to any associated changes in the ECF volume (Box 16.12). In all cases, there is retention of water relative to sodium, and it is clinical examination rather than the biochemical results that gives a clue to the underlying cause. Patients who have hyponatraemia in association with a sodium deficit (‘depletional hyponatraemia’) have clinical features of hypovolaemia (see Box 16.4, p. 433) and supportive laboratory findings, including low urinary sodium concentration (< 30 mmol/L) and elevated plasma renin activity. The cause of sodium loss is usually apparent; common examples are shown in Box 16.12. Water retention also occurs in the syndrome of inappropriate secretion of ADH (SIADH). In this condition, an endogenous source of ADH (either cerebral or tumour-derived) promotes water retention by the kidney in the absence of an appropriate physiological stimulus (Box 16.13). The clinical diagnosis requires the patient to be euvolaemic, with no evidence of cardiac, renal or hepatic disease potentially associated with hyponatraemia. Other non-osmotic stimuli that cause release of ADH (pain, stress, nausea) should also be excluded. Supportive laboratory findings are shown in Box 16.13. In this situation, plasma concentrations of sodium, chloride, urea and uric acid are low with a correspondingly reduced osmolality. Urine osmolality, which should physiologically be maximally dilute (approximately 50 mmol/kg) in the face of low plasma osmolality, is higher than at least 100 mmol/kg and indeed is typically higher than the plasma osmolality. The urine sodium concentration is typically high (> 30 mmol/L), consistent with euvolaemia and lack of compensatory factors promoting sodium retention.
Clinical biochemistry and metabolism
Biochemical investigations
Integrated water and electrolyte balance
Water and electrolyte distribution
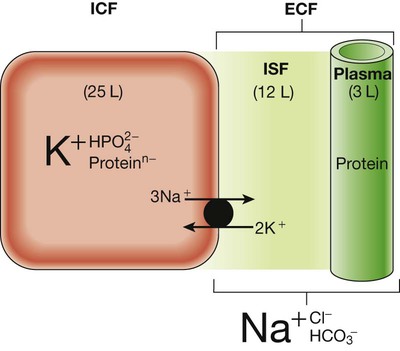
Schematic representation of volume (L = litres) and composition (dominant ionic species only shown) of the intracellular fluid (ICF) and extracellular fluid (ECF) in a 70 kg male. The main difference in composition between the plasma and interstitial fluid (ISF) is the presence of appreciable concentrations of protein in the plasma but not the ISF.
Investigation of water and electrolytes
Disorders of sodium balance
Functional anatomy and physiology of renal sodium handling
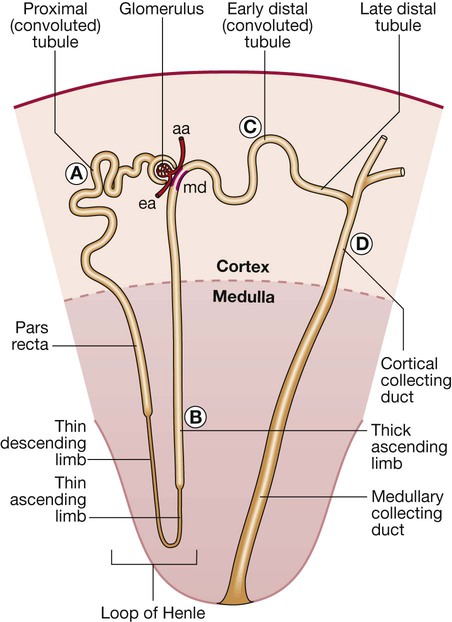
Letters A–D refer to tubular segments shown in more detail in Figure 16.3. (aa = afferent arteriole; ea = efferent arteriole; md = macula densa)
Nephron segments
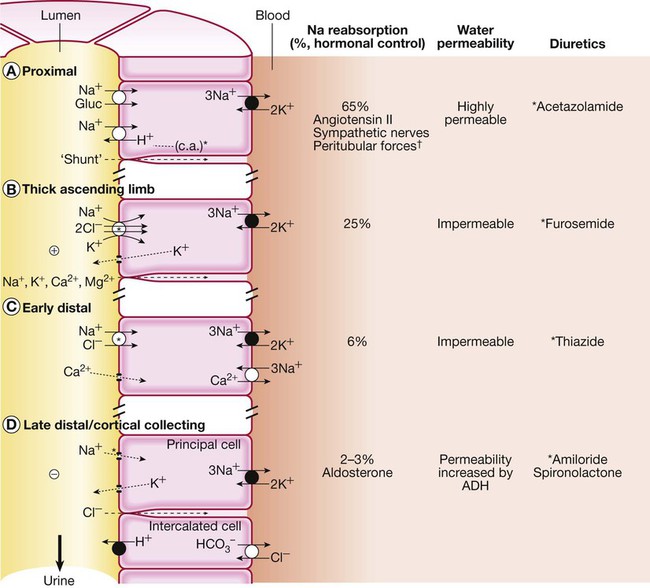
See text for further details. The apical membrane of the tubular cells is the side facing the lumen, and the basolateral membrane is the side facing the blood. Black circles indicate primary active transport pumps; open circles are carrier molecules without direct linkage to adenosine triphosphate (ATP) hydrolysis. *Site of diuretic action in each segment. †Peritubular forces refers to the hydrostatic and oncotic pressures in the peritubular capillaries. (ADH = antidiuretic hormone; c.a. = carbonic anhydrase)
Proximal tubule
The loop of Henle
Early distal tubule
Late distal tubule and collecting ducts
Regulation of sodium transport
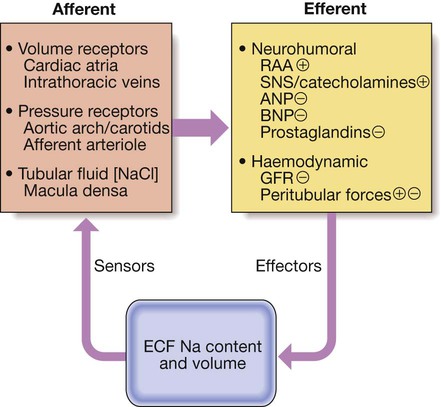
(ANP = atrial natriuretic peptide; BNP = brain natriuretic peptide; ECF = extracellular fluid; GFR = glomerular filtration rate; RAA = renin–angiotensin–aldosterone system; SNS = sympathetic nervous system. ⨁ indicates an effect to stimulate Na reabsorption and hence reduce Na excretion, while  indicates an effect to inhibit Na reabsorption and hence increase Na excretion)
indicates an effect to inhibit Na reabsorption and hence increase Na excretion)
Presenting problems in disorders of sodium balance
Sodium depletion
Aetiology and clinical assessment
Management
Intravenous fluid therapy
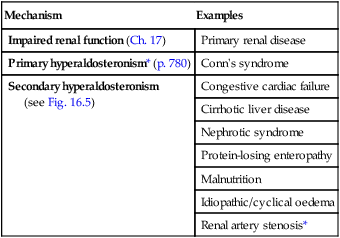
Sodium excess
Aetiology and clinical assessment
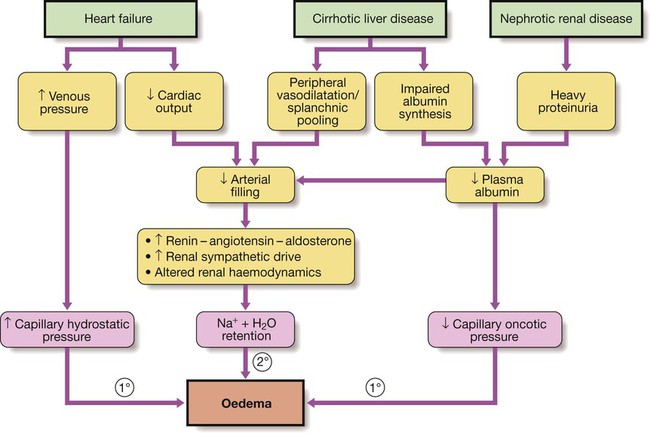
Primary renal retention of Na and water may also contribute to oedema formation when GFR is significantly reduced (see Box 16.10 and p. 478).
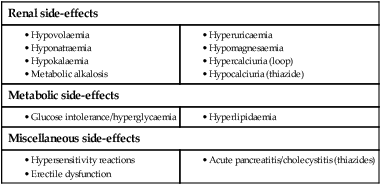
Diuretic therapy
Mechanisms of action
Clinical use of diuretics
Disorders of water balance
Functional anatomy and physiology of renal water handling
Presenting problems in disorders of water balance
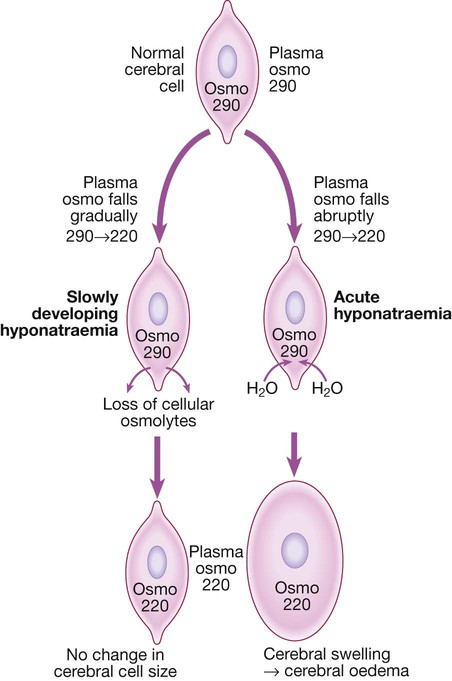
Hyponatraemia
Aetiology and clinical assessment
Hyponatraemia with hypovolaemia
Hyponatraemia with euvolaemia