52
Blood & Tissue Protozoa
CHAPTER CONTENTS
INTRODUCTION
The medically important organisms in this category of protozoa consist of the sporozoans Plasmodium and Toxoplasma and the flagellates Trypanosoma and Leishmania. Pneumocystis is discussed in this book as a protozoan because it is considered as such from a medical point of view. However, molecular data indicate that it is related to yeasts such as Saccharomyces cerevisiae. Table 51–2 summarizes several important features of these blood and tissue protozoa.
The medically important stages in the life cycle of the blood and tissue protozoa are described in Table 52–1.
PLASMODIUM
Disease
Malaria is caused by four plasmodia: Plasmodium vivax, Plasmodium ovale, Plasmodium malariae, and Plasmodium falciparum. P. vivax and P. falciparum are more common causes of malaria than are P. ovale and P. malariae. Worldwide, malaria is one of the most common infectious diseases and a leading cause of death.
Important Properties
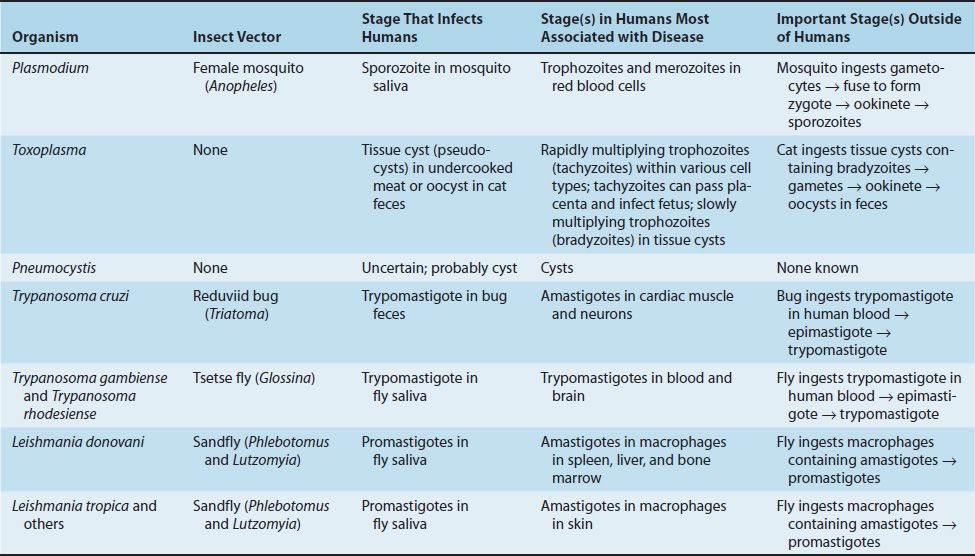
The life cycle of Plasmodium species is shown in Figure 52–1. The vector and definitive host for plasmodia is the female Anopheles mosquito (only the female takes a blood meal). There are two phases in the life cycle: the sexual cycle, which occurs primarily in mosquitoes, and the asexual cycle, which occurs in humans, the intermediate hosts.1
FIGURE 52–1 Plasmodium species. Life cycle. Right side of figure describes the stages within the human (blue arrows). Cycle A (top right) is the exo-erythrocyte stage that occurs in the liver. Cycle B (bottom right) is the erythrocyte stage that occurs in the red blood cell. Note that at step 6 in the cycle, merozoites released from the ruptured schizonts then infect other red blood cells. The synchronized release of merozoites causes the periodic fever and chills characteristic of malaria. Left side of figure describes the stages within the mosquito (red arrows). Humans are infected at step 1 when mosquito injects sporozoites. Mosquito is infected at step 8 when mosquito ingests gametocytes in human blood. (Provider: Centers for Disease Control and Prevention/Dr. Alexander J. da Silva and Melanie Moser.)
The sexual cycle is called sporogony because sporozoites are produced (sporogonic cycle is labeled C in Figure 52–1), and the asexual cycle is called schizogony because schizonts are made.
The life cycle in humans begins with the introduction of sporozoites into the blood from the saliva of the biting mosquito. The sporozoites are taken up by hepatocytes within 30 minutes. This “exoerythrocytic” phase (labeled A in Figure 52–1) consists of cell multiplication and differentiation into merozoites. P. vivax and P. ovale produce a latent form (hypnozoite) in the liver; this form is the cause of relapses seen with vivax and ovale malaria.
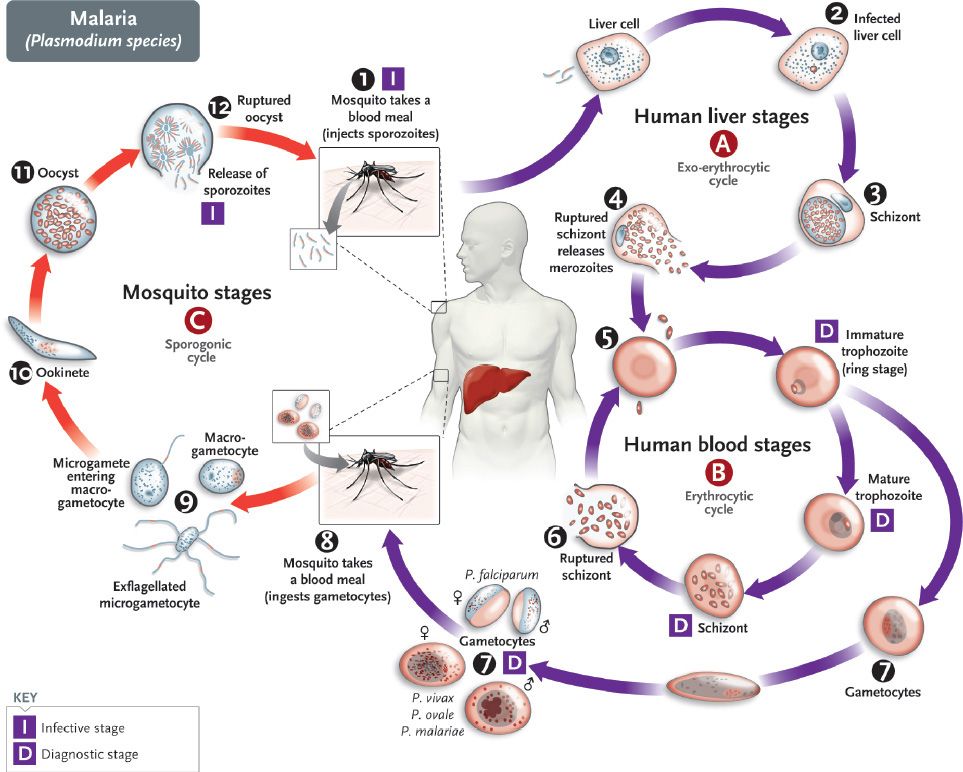
Merozoites are released from the liver cells and infect red blood cells. During the erythrocytic phase (labeled B in Figure 52–1), the organism differentiates into a ring-shaped trophozoite (Figures 52–2A and B and 52–3). The ring form grows into an ameboid form and then differentiates into a schizont filled with merozoites (Figure 52–2C). After release, the merozoites infect other erythrocytes (step 6 in Figure 52–1). This cycle in the red blood cell repeats at regular intervals typical for each species. The periodic release of merozoites causes the typical recurrent symptoms of chills, fever, and sweats seen in malaria patients.
FIGURE 52–2 A: Plasmodium vivax signet-ring trophozoite within a red blood cell. B: Plasmodium vivax ameboid trophozoite within a red blood cell, showing Schüffner’s dots. C: Plasmodium vivax mature schizont with merozoites inside. D: Plasmodium vivax microgametocyte. E: Plasmodium vivax macrogametocyte. F: Plasmodium falciparum “banana-shaped” gametocyte with attached red cell ghost. G: Toxoplasma gondii trophozoites within macrophage. H: Pneumocystis jiroveci cysts. (A–G, 1200×; H, 800×.)
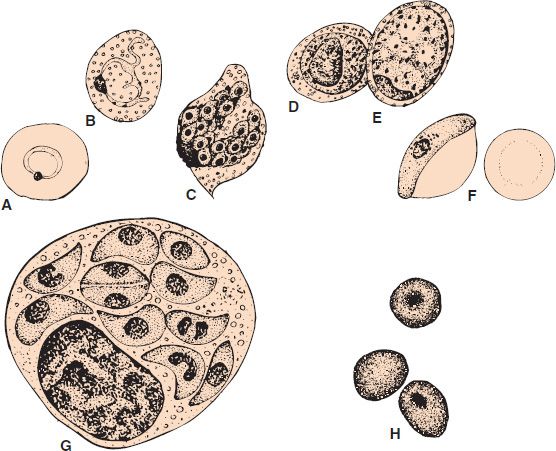
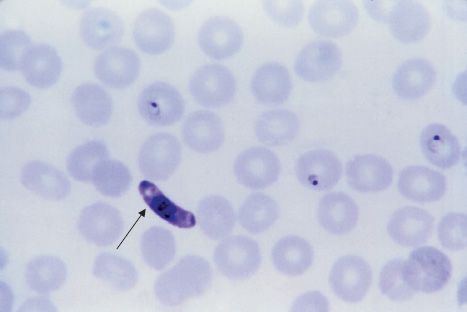
FIGURE 52–3 Plasmodium falciparum—ring-shaped trophozoite. Long arrow points to a red blood cell containing a ring-shaped trophozoite. Arrowhead points to a red blood cell containing four ring-shaped trophozoites. Note the very high percentage of red cells containing ring forms. This high-level parasitemia is more often seen in Plasmodium falciparum infection than in infection by the other plasmodia. (Figure courtesy of Dr. S. Glenn, Public Health Image Library, Centers for Disease Control and Prevention.)
The sexual cycle begins in the human red blood cells when some merozoites develop into male and others into female gametocytes (Figures 52–2D to F and 52–4, and step 7 in Figure 52–1). The gametocyte-containing red blood cells are ingested by the female Anopheles mosquito and, within her gut, produce a female macrogamete and eight spermlike male microgametes. After fertilization, the diploid zygote differentiates into a motile ookinete that burrows into the gut wall, where it grows into an oocyst within which many haploid sporozoites are produced. The sporozoites are released and migrate to the salivary glands, ready to complete the cycle when the mosquito takes her next blood meal.
FIGURE 52–4 Plasmodium falciparum—gametocyte. Arrow points to a “banana-shaped” gametocyte of Plasmodium falciparum. (Figure courtesy of Dr. S. Glenn, Public Health Image Library, Centers for Disease Control and Prevention.)
A very important feature of P. falciparum is chloroquine resistance. Chloroquine-resistant strains now predominate in most areas of the world where malaria is endemic. Chloroquine resistance is mediated by a mutation in the gene encoding the chloroquine transporter in the cell membrane of the organism.
Pathogenesis & Epidemiology
Most of the pathologic findings of malaria result from the destruction of red blood cells. Red cells are destroyed both by the release of the merozoites and by the action of the spleen to first sequester the infected red cells and then to lyse them. The enlarged spleen characteristic of malaria is due to congestion of sinusoids with erythrocytes, coupled with hyperplasia of lymphocytes and macrophages.
Malaria caused by P. falciparum is more severe than that caused by other plasmodia. It is characterized by infection of far more red cells than the other malarial species and by occlusion of the capillaries with aggregates of parasitized red cells. This leads to life-threatening hemorrhage and necrosis, particularly in the brain (cerebral malaria). Furthermore, extensive hemolysis and kidney damage occur, with resulting hemoglobinuria. The dark color of the patient’s urine has given rise to the term “blackwater fever.” The hemoglobinuria can lead to acute renal failure.
The timing of the fever cycle is 72 hours for P. malariae and 48 hours for the other plasmodia. Disease caused by P. malariae is called quartan malaria because it recurs every fourth day, whereas malaria caused by the other plasmodia is called tertian malaria because it recurs every third day. Tertian malaria is subdivided into malignant malaria, caused by P. falciparum, and benign malaria, caused by P. vivax and P. ovale.
P. falciparum causes a high level of parasitemia because it can infect red cells of all ages. In contrast, P. vivax infects only reticulocytes and P. malariae infects only mature red cells; therefore, they produce much lower levels of parasites in the blood. Individuals with sickle cell trait (heterozygotes) are protected against malaria because their red cells have too little ATPase activity and cannot produce sufficient energy to support the growth of the parasite. People with homozygous sickle cell anemia are also protected but rarely live long enough to obtain much benefit.
The receptor for P. vivax is the Duffy blood group antigen. People who are homozygous recessive for the genes that encode this protein are resistant to infection by P. vivax. More than 90% of black West Africans and many of their American descendants do not produce the Duffy antigen and are thereby resistant to vivax malaria.
People with glucose-6-phosphate dehydrogenase (G6PD) deficiency are also protected against the severe effects of falciparum malaria. G6PD deficiency is an X-linked hemoglobinopathy found in high frequency in tropical areas where malaria is endemic. Both male and female carriers of the mutated gene are protected against malaria.
Malaria is transmitted primarily by mosquito bites, but transmission across the placenta, in blood transfusions, and by intravenous drug use also occurs.
Partial immunity based on humoral antibodies that block merozoites from invading the red cells occurs in infected individuals. A low level of parasitemia and low-grade symptoms result; this condition is known as premunition. In contrast, a nonimmune person, such as a first-time traveler to an area where falciparum malaria is endemic, is at risk of severe, life-threatening disease.
More than 200 million people worldwide have malaria, and more than 1 million die of it each year, making it the most common lethal infectious disease. It occurs primarily in tropical and subtropical areas, especially in Asia, Africa, and Central and South America. Malaria in the United States is seen in Americans who travel to areas of endemic infection without adequate chemoprophylaxis and in immigrants from areas of endemic infection. It is not endemic in the United States. Certain regions in Southeast Asia, South America, and east Africa are particularly affected by chloroquine-resistant strains of P. falciparum. People who have lived or traveled in areas where malaria occurs should seek medical attention for febrile illnesses up to 3 years after leaving the malarious area.
Clinical Findings
Malaria presents with abrupt onset of fever and chills, accompanied by headache, myalgias, and arthralgias, about 2 weeks after the mosquito bite. Fever may be continuous early in the disease; the typical periodic cycle does not develop for several days after onset. The fever spike, which can reach 41°C, is frequently accompanied by shaking chills, nausea, vomiting, and abdominal pain. The fever is followed by drenching sweats. Patients usually feel well between the febrile episodes. Splenomegaly is seen in most patients, and hepatomegaly occurs in roughly one-third. Anemia is prominent.
Untreated malaria caused by P. falciparum is potentially life-threatening as a result of extensive brain (cerebral malaria) and kidney (blackwater fever) damage. Malaria caused by the other three plasmodia is usually self-limited, with a low mortality rate. However, relapses of P. vivax and P. ovale malaria can occur up to several years after the initial illness as a result of hypnozoites latent in the liver.
Laboratory Diagnosis
Diagnosis rests on microscopic examination of blood, using both thick and thin Giemsa-stained smears. The thick smear is used to screen for the presence of organisms, and the thin smear is used for species identification. It is important to identify the species because the treatment of different species can differ. Ring-shaped trophozoites can be seen within infected red blood cells (Figure 52–3). The gametocytes of P. falciparum are crescent-shaped (“banana-shaped”), whereas those of the other plasmodia are spherical (Figure 52–2F). If more than 5% of red blood cells are parasitized, the diagnosis is usually P. falciparum malaria.
If blood smears do not reveal the diagnosis, then a polymerase chain reaction (PCR)-based test for Plasmodium nucleic acids or an enzyme-linked immunosorbent assay (ELISA) test for a protein specific for P. falciparum can be useful.
Treatment
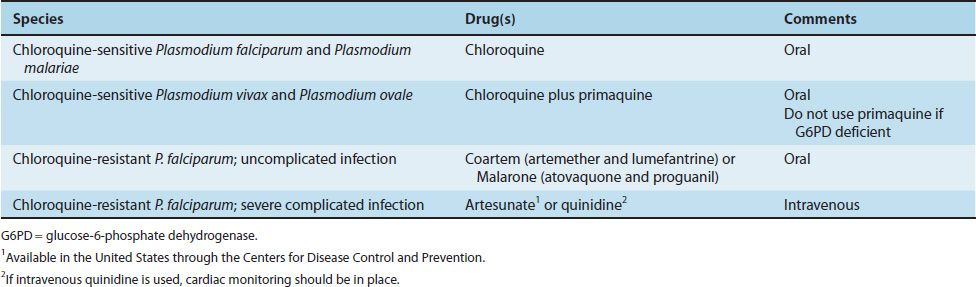
The treatment of malaria is complicated, and the details are beyond the scope of this book. Table 52–2 presents the drugs commonly used in the United States. The main criteria used for choosing specific drugs are the severity of the disease and whether the organism is resistant to chloroquine. Chloroquine resistance is determined by the geographical location where the infection was acquired rather than by laboratory testing.
Chloroquine is the drug of choice for treatment of uncomplicated malaria caused by non-falciparum species in areas without chloroquine resistance. Chloroquine kills the merozoites, thereby reducing the parasitemia, but does not affect the hypnozoites of P. vivax and P. ovale in the liver. These are killed by primaquine, which must be used to prevent relapses. Primaquine may induce severe hemolysis in those with G6PD deficiency, so testing for this enzyme should be done before the drug is given. Primaquine should not be given if the patient is severely G6PD deficient. If primaquine is not given, one approach is to wait to see whether symptoms recur and then treat with chloroquine.
Uncomplicated, chloroquine-resistant P. falciparum infection is treated with either Coartem (artemether plus lumefantrine) or Malarone (atovaquone and proguanil). In severe complicated cases of chloroquine-resistant falciparum malaria, intravenous administration of either artesunate or quinidine is used.
Outside the United States, the artemisinins, such as artesunate or artemether, are widely used in combination with other antimalarial drugs. The artemisinins are inexpensive and have few side effects, and most plasmodia have not developed resistance to these drugs. However, resistance to artesunate has emerged in some strains of P. falciparum in Southeast Asia (e.g., Cambodia, Myanmar, and Thailand).
Prevention
Chemoprophylaxis of malaria for travelers to areas where chloroquine-resistant P. falciparum is endemic consists of mefloquine or doxycycline. A combination of atovaquone and proguanil (Malarone), in a fixed dose, can also be used. Chloroquine should be used in areas where P. falciparum is sensitive to that drug. Travelers to areas where the other three plasmodia are found should take chloroquine starting 2 weeks before arrival in the endemic area and continuing for 6 weeks after departure. This should be followed by a 2-week course of primaquine if exposure was high. Primaquine will kill the hypnozoites of P. vivax and P. ovale.
Other preventive measures include the use of mosquito netting, window screens, protective clothing, and insect repellents. The mosquitoes feed from dusk to dawn, so protection is particularly important during the night. Communal preventive measures are directed against reducing the mosquito population. Many insecticide sprays, such as DDT, are no longer effective because the mosquitoes have developed resistance. Drainage of stagnant water in swamps and ditches reduces the breeding areas. There is no vaccine.
TOXOPLASMA
Disease
Toxoplasma gondii causes toxoplasmosis, including congenital toxoplasmosis.
Important Properties
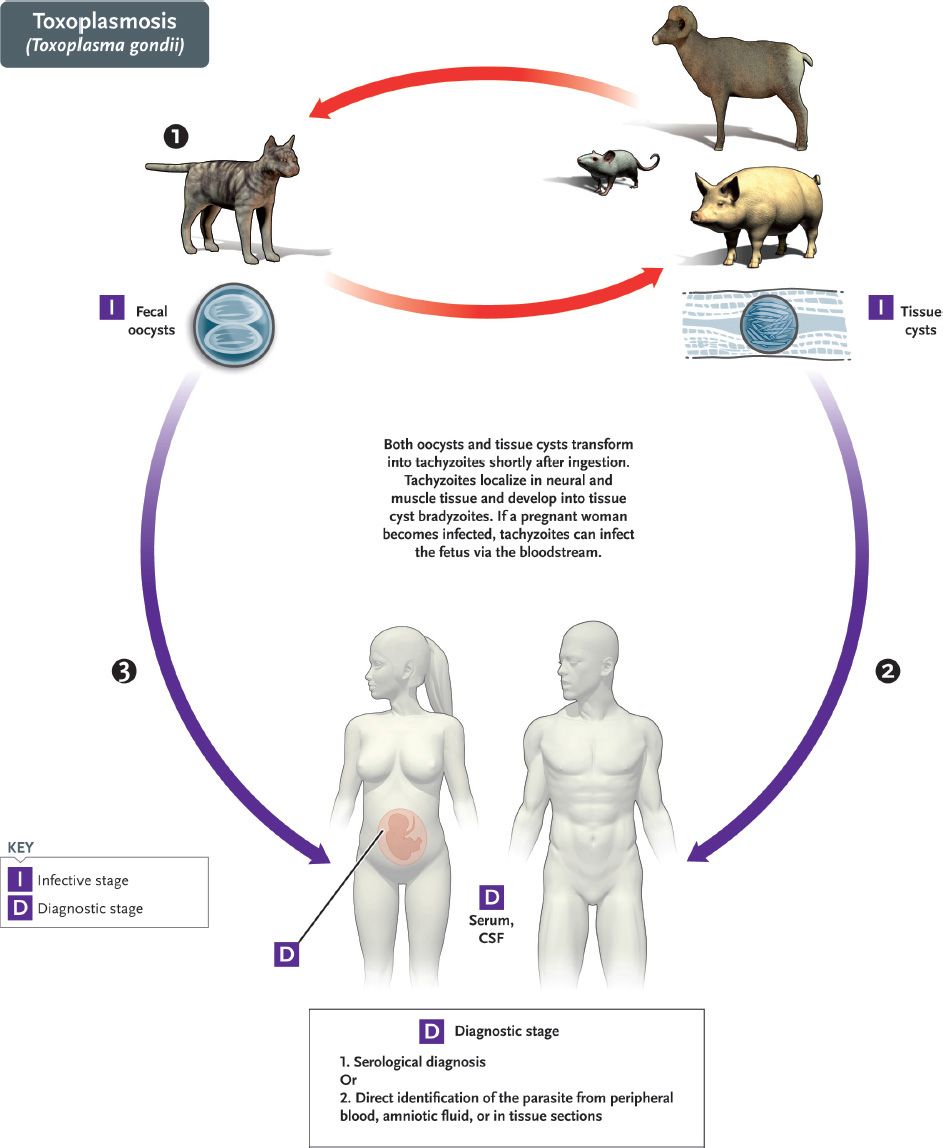
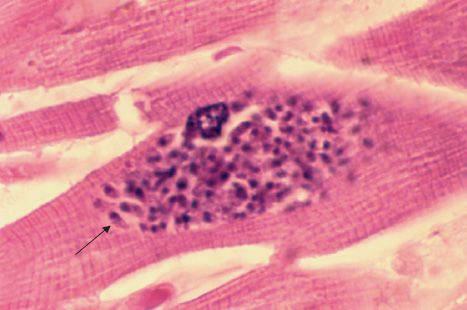
The life cycle of T. gondii is shown in Figure 52–5. The definitive host is the domestic cat and other felines; humans and other mammals are intermediate hosts. Infection of humans begins with the ingestion of cysts in undercooked meat or from accidental contact with cysts in cat feces. In the small intestine, the cysts rupture and release forms that invade the gut wall, where they are ingested by macrophages and differentiate into rapidly multiplying trophozoites (tachyzoites), which kill the cells and infect other cells (Figures 52–2G and 52–6). Cell-mediated immunity usually limits the spread of tachyzoites, and the parasites enter host cells in the brain, muscle, and other tissues, where they develop into cysts in which the parasites multiply slowly. These forms are called bradyzoites. These tissue cysts are both an important diagnostic feature and a source of organisms when the tissue cyst breaks in an immunocompromised patient.
FIGURE 52–5 Toxoplasma gondii. Life cycle. Top red arrows show the natural life cycle as T. gondii circulates between cats (#1), which excrete oocysts in the feces that are eaten by mice, but also by domestic animals such as pigs and sheep. Cysts form in tissue such as muscle and brain. The natural cycle is completed when cats eat mice. Humans are accidental hosts. They can be infected by the ingestion of under-cooked pork and lamb (blue arrow #2) containing tissue cysts in muscle or by ingestion of food contaminated with cat feces containing oocysts (blue arrow #3). (Provider: Centers for Disease Control and Prevention/Dr. Alexander J. da Silva and Melanie Moser.)
FIGURE 52–6 Toxoplasma gondii—tachyzoite. Arrow points to a tachyzoite of T. gondii in cardiac muscle. (Figure courtesy of Dr. E. Ewing, Jr., Public Health Image Library, Centers for Disease Control and Prevention.)
The cycle within the cat begins with the ingestion of cysts in raw meat (e.g., mice). Bradyzoites are released from the cysts in the small intestine, infect the mucosal cells, and differentiate into male and female gametocytes, whose gametes fuse to form oocysts that are excreted in cat feces. The cycle is completed when soil contaminated with cat feces is accidentally ingested. Human infection usually occurs from eating undercooked meat (e.g., lamb and pork) from animals that grazed in soil contaminated with infected cat feces.
Pathogenesis & Epidemiology
T. gondii is usually acquired by ingestion of cysts in uncooked meat or cat feces.
Transplacental transmission from an infected mother to the fetus occurs also. Human-to-human transmission, other than transplacental transmission, does not occur. After infection of the intestinal epithelium, the organisms spread to other organs, especially the brain, lungs, liver, and eyes. Progression of the infection is usually limited by a competent immune system. Cell-mediated immunity plays the major role, but circulating antibody enhances killing of the organism. Most initial infections are asymptomatic. When contained, the organisms persist as cysts within tissues. There is no inflammation, and the individual remains well unless immunosuppression allows activation of organisms in the cysts.
Congenital infection of the fetus occurs only when the mother is infected during pregnancy. If she is infected before the pregnancy, the organism will be in the cyst form and there will be no trophozoites to pass through the placenta. The mother who is reinfected during pregnancy but who has immunity from a previous infection will not transmit the organism to her child. Roughly one-third of mothers infected during pregnancy give birth to infected infants, but only 10% of these infants are symptomatic.
Infection by T. gondii occurs worldwide. Serologic surveys reveal that in the United States antibodies are found in 5% to 50% of people in various regions. Infection is usually sporadic, but outbreaks associated with ingestion of raw meat or contaminated water occur. Approximately 1% of domestic cats in the United States shed Toxoplasma cysts.
Clinical Findings
Most primary infections in immunocompetent adults are asymptomatic, but some resemble infectious mononucleosis, except that the heterophil antibody test is negative. Congenital infection can result in abortion, stillbirth, or neonatal disease with encephalitis, chorioretinitis, and hepatosplenomegaly. Fever, jaundice, and intracranial calcifications are also seen. Most infected newborns are asymptomatic, but chorioretinitis or mental retardation will develop in some children months or years later. Congenital infection with Toxoplasma is one of the leading causes of blindness in children. In patients with reduced cell-mediated immunity (e.g., patients with acquired immunodeficiency syndrome [AIDS]), life-threatening disseminated disease, primarily encephalitis, occurs.
Laboratory Diagnosis
For the diagnosis of acute and congenital infections, an immunofluorescence assay for IgM antibody is used. IgM is used to diagnose congenital infection, because IgG can be maternal in origin. Tests of IgG antibody can be used to diagnose acute infections if a significant rise in antibody titer in paired sera is observed.
Microscopic examination of Giemsa-stained preparations shows crescent-shaped trophozoites during acute infections. Cysts may be seen in the tissue. The organism can be grown in cell culture. Inoculation into mice can confirm the diagnosis.
Treatment
Congenital toxoplasmosis, whether symptomatic or asymptomatic, should be treated with a combination of sulfadiazine and pyrimethamine. These drugs also constitute the treatment of choice for disseminated disease in immunocompromised patients. Acute toxoplasmosis in an immunocompetent individual is usually self-limited, but any patient with chorioretinitis should be treated.
Prevention
The most effective means of preventing toxoplasmosis is to cook meat thoroughly to kill the cysts. Pregnant women should be especially careful to avoid undercooked meat and contact with cat feces. They should refrain from emptying cat litter boxes. Cats should not be fed raw meat. Trimethoprim-sulfamethoxazole is used to prevent Toxoplasma encephalitis in patients infected with human immunodeficiency virus (HIV).
PNEUMOCYSTIS
Disease
Pneumocystis jiroveci is an important cause of pneumonia in immunocompromised individuals. In 2002, taxonomists renamed the human species of Pneumocystis as P. jiroveci and recommended that Pneumocystis carinii be used only to describe the rat species of Pneumocystis.
Important Properties
The classification and life cycle of Pneumocystis are unclear. Many aspects of its biochemistry indicate that it is a yeast, but it also has several attributes of a protozoan. An analysis of rRNA sequences published in 1988 indicates that Pneumocystis should be classified as a fungus related to yeasts such as Saccharomyces cerevisiae. Subsequent analysis of mitochondrial DNA and of various enzymes supports the idea that it is a fungus. However, it does not have ergosterol in its membranes as do the fungi. It has cholesterol.
Medically, it is still thought of as a protozoan. In tissue, it appears as a cyst that resembles the cysts of protozoa (Figures 52–2H and 52–7). The findings that it does not grow on fungal media and that antifungal drugs are ineffective have delayed acceptance of its classification as a fungus.
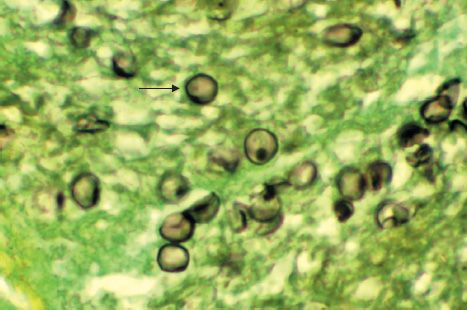
FIGURE 52–7 Pneumocystis jiroveci—arrow points to a cyst of P. jiroveci in lung tissue. (Figure courtesy of Dr. E. Ewing, Jr., Public Health Image Library, Centers for Disease Control and Prevention.)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree