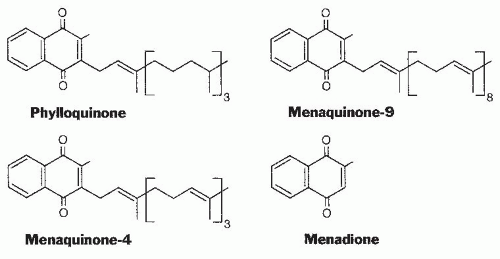
Analysis, Food Content, and Bioavailability
Standardized procedures suitable for the assay of the vitamin K content of foods are available, and sufficient values have been obtained (
7) to provide a reasonable estimate of dietary intake of the vitamin (
Table 20.1). Green, leafy vegetables are the foods with the highest phylloquinone content in most diets. Foods providing substantial amounts of the vitamin to most of the population are spinach (380 µg/100 g), broccoli (180 µg/100 g), and iceberg lettuce (35 µg/100 g). Fats and oils also contribute to the daily vitamin K intake of many individuals.
The phylloquinone content of oils varies considerably; soybean oil (190 µg/100 g) and canola oil (130 µg/100 g) have a high content, and corn oil (3 µg/100 g) is a poor source. The source of fat or oil has a major influence on the vitamin K content of margarine and prepared foods with a high fat content. The process of hydrogenation to convert plant oils to solid margarines or shortening converts some of the phylloquinone to 2′,3′-dihydrophylloquinone with a completely saturated side chain. The biologic activity of this form of the vitamin is lower than that of phylloquinone but has not been accurately determined. Investigators have found that the intake of this form of the vitamin by the US population is 20% to 25% that of phylloquinone (
8).
The bioavailability of phylloquinone from various foods in human subjects has been difficult to assess. Initial studies compared the increase in plasma phylloquinone from the consumption of green vegetables with that of pure phylloquinone. These limited studies suggested that the bioavailability of phylloquinone from various vegetable
sources should not be considered more than 15% to 20% as available as phylloquinone consumed as a supplement. The availability of phylloquinone from vegetable oil added to corn oil was found to be about twice that from broccoli. The use of stable isotopes labeled phylloquinone should result in more accurate measurements of bioavailability (
9,
10,
11). These findings demonstrate that meal composition is an important factor (
12).
A limited number of foods, mainly cheeses, do contain a significant (50 to 70 µg/100 g) amount of long-chain MKs, and a fermented soybean product, natto, that is consumed mainly in the Japanese market contains nearly 1000 µg/100 g of MK-7. Limited data indicate that the absorption of longchain MKs may be substantially higher than the absorption of phylloquinone from green vegetables (
13).
Absorption and Transport of Vitamin K
Phylloquinone, the predominant dietary form of the vitamin, is absorbed from the intestine through the lymphatic system (
14), and absorption is decreased in patients with biliary insufficiency or various malabsorption syndromes. Phylloquinone in plasma is predominantly carried by the triglyceride-rich lipoprotein fraction containing very-lowdensity lipoproteins and chylomicron remnants, although some is located in the low-density lipoprotein and highdensity lipoprotein fractions (
15). Plasma phylloquinone concentrations in a physiologically normal population have been shown to have a mean of approximately 1.0 nmol/L (˜0.45 ng/mL), with a wide range in values from 0.3 to 2.6 nmol/L (
16). As expected from this route of transport, plasma phylloquinone concentrations are strongly correlated with plasma lipid levels (
17).
The major route of entry of phylloquinone into tissues appears to be through clearance of chylomicron remnants by apolipoprotein E (ApoE) receptors. The polymorphism of ApoE influences fasting plasma phylloquinone concentrations. This response is correlated with the hepatic clearance of chylomicron remnants from the circulation, with ApoE2 having the slowest rate of removal (
18). The secretion of phylloquinone from the liver and the process by which the vitamin moves among organs are not yet understood.
The total human body pool of phylloquinone is very small, and turnover is rapid. A peak of circulating phylloquinone concentration following absorption has been shown to be rapidly decreased (half-life ˜15 minutes), followed by a slower decrease (half-life ˜2.5 hours) (
10). Although the total amount of vitamin K is relatively high, long-chain MKs, rather than phylloquinone, are the major source of the vitamin in liver (
2). Data based on liver biopsies of patients fed diets very low in vitamin K before surgery indicate that approximately two thirds of hepatic phylloquinone was lost in 3 days (
19). These findings are consistent with a small pool size of phylloquinone that turns over very rapidly. The large amount of MKs in the liver, however, turns over at a much lower rate.
The major route of ingested phylloquinone excretion is through the feces, and very little unmetabolized vitamin is excreted. Many details of the metabolic transformation of the vitamin are currently lacking, but investigators have shown that the side chains of phylloquinone and MK-4 are shortened to seven or five carbon atoms yielding a carboxylic acid group at the end (
14,
20). These
5C and
7C-aglycones, which are the major metabolites of phylloquinone, are excreted in the urine at concentrations that are related to the intake of the vitamin (
21). Studies have also shown that glucuronides of menadione are excreted in urine at an amount that is positively related to phylloquinone (
22). The mechanism by which menadione is cleaved from various sources of vitamin K or its metabolites is not known. Evidence indicates the existence of numerous other unidentified metabolites, and it also shows that treatment of patients with warfarin, which results in a substantial conversion of the body pool of phylloquinone to phylloquinone-2,3-epoxide, leads to the generation of new metabolites.
Utilization of Menaquinones from the Large Bowel
Substantial amounts of vitamin K in the form of longchain MKs are known to be present in the human gut. Relatively few of the bacteria that comprise the normal intestinal flora are major producers of MKs. Obligate anaerobes of the
Bacteroides (B. fragilis), Eubacterium, Propionibacterium, and
Arachnia genera are major producers, however, as are facultatively anaerobic organisms such as
Escherichia coli. The amount of vitamin K in the gut can be quite large, and the amounts found in total intestinal tract contents from five patients who underwent colonoscopy ranged from 0.3 to 5.1 mg (
23), with MK-9 and MK-10 the major contributors. These amounts are considerably larger than the daily dietary requirement for the vitamin, which is less than 100 µg/day. Long-chain MKs, mainly MK-6, MK-7, MK-10, and MK-11, are present at very low levels in plasma, but they have been found in human liver at levels that greatly exceed the phylloquinone concentration (
24).
A major question remaining is how these very lipophilic compounds that are present as constituents of bacterial membranes are absorbed from the lower bowel. Little evidence on the route of absorption and transport of these vitamins to the liver is available.
Vitamin K deficiency in the adult human that is characterized by vitamin K-responsive hypoprothrombinemia is a very rare condition, and numerous case reports of antibiotic-induced hypoprothrombinemia are often cited as evidence of the importance of bacterial MKs. These antibiotic-induced hypoprothrombinemias have historically been presumed to result from a decrease in the synthesis of MKs by gut organisms (
25), with the underlying assumption that MKs are important in satisfying at least a portion of the normal human requirement for vitamin K. In nearly all these case reports, however, evidence of
decreased MK synthesis in the presence of antibiotic treatment is lacking, and the drugs themselves may have influenced hemostatic control. The difficulty in producing a clinically significant deficiency in human subjects, such as an increased prothrombin time (PT) by dietary restriction, and the known rapid turnover of the body phylloquinone pool strongly suggest that MKs do contribute to maintaining adequate vitamin K status (
24), but the magnitude of the contribution cannot be determined with the available data.