HISTORICAL OVERVIEW
In 1922, Evans and Bishop (
1), during their investigations of infertility, first described fetal resorption as a symptom of vitamin E deficiency in rats fed “rancid lard.” In 1936, Evans et al (
2) isolated a factor from wheat germ and named it “α-tocopherol,” a name derived from the Greek “tokos” (offspring) and “pherein” (to bear), with an “ol” to indicate that it was an alcohol. Two other tocopherols, β- and γ-, with lower biologic activities, were isolated from vegetable oils (
3). These early observations formed the foundation for defining the “biologic activity” of vitamin E, which is based on its ability to prevent or reverse specific vitamin E deficiency symptoms (
4). Now it is recognized that the various forms are not interconvertible, and only α-tocopherol meets human requirements (
5).
Vitamin E deficiency symptoms in various animal species were reviewed by Machlin (
4). Necrotizing myopathy, fetal death and resorption, anemia, and accumulation of lipofuscin (a fluorescent pigment of “aging”) in tissues have been observed in vitamin E-deficient animals. Progressive, peripheral, sensory neuropathy is the first sign of vitamin E deficiency in humans (
6).
Horwitt et al (
7,
8) attempted to induce vitamin E deficiency in men by feeding a diet low in vitamin E for 6 years to volunteers at the Elgin (Illinois) State Hospital in the 1950s. These data were used in 2000 to set the recommended dietary allowance (RDA) for vitamin E (
5), discussed later.
It was not until the mid-1960s that vitamin E deficiency was described in children with fat malabsorption syndromes, as reviewed (
9). Subsequently, vitamin E-deficient patients with peripheral neuropathies but without fat malabsorption were described (
10). Studies in such patients opened new avenues in vitamin E investigations because these patients were found to have a genetic defect in the hepatic α-tocopherol transfer protein (α-TTP) (
11,
12).
TERMINOLOGY
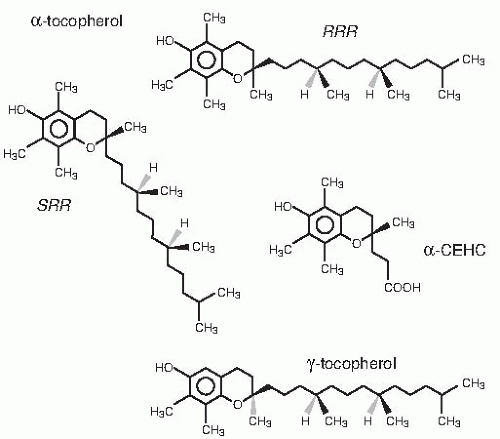
The Institute of Medicine (IOM), Food and Nutrition Board, defined that only α-tocopherol meets human vitamin E requirements (
5). Molecules with α-tocopherol antioxidant activity include four tocopherols and four
tocotrienols (
Fig. 19.1), however. These molecules have similar chromanol structures: trimethyl (α-), dimethyl (β- or γ-), and monomethyl (δ-); tocopherols have a phytyl side chain, whereas tocotrienols have an unsaturated side chain. α-Tocopherol synthesized by condensation of trimethyl hydroquinone with racemic isophytol (
13) contains eight stereoisomers (arising from the three chiral centers: 2′, 4′, and 8′, specifically:
RRR, RSR, RSS, RRS, SRR, SSR, SRS, and
SSS) and is designated
all-rac-α-tocopherol (incorrectly called
dl-α-tocopherol) (see
Fig. 19.1). The naturally occurring
RRR-α-tocopherol (formerly called
d-α-tocopherol) is only one of the eight stereoisomers present in
all-rac-α-tocopherol. The IOM (
5) defined that human vitamin E requirements are met only by 2
R-α-tocopherols, that is, half of the stereoisomers in
all-rac-α-tocopherol. Previously, γ-tocopherol and other vitamin E forms were included as sources of vitamin E; these forms no longer are included because of a lack of evidence showing they have health benefits in humans (
5).
The IOM definition of vitamin E has led to confusion about vitamin E units. The definition of the unit used on supplement labels derives from units set by the US Pharmacopoeia (
14). These supplements often contain esters of α-tocopherol, such as α-tocopheryl acetate, succinate, or nicotinate. Previously, the vitamin E international unit (IU) was defined as 1 IU = 1 mg
all-rac-α-tocopheryl acetate or 0.67 mg
RRR-α-tocopherol, or 0.74 mg
RRR-α-tocopheryl acetate. The IOM (see
Table 6.1 in reference 5), however, defined the vitamin E requirement in milligrams of 2
R-α-tocopherol and provided conversion factors, such that 1 mg
all-rac-α-tocopherol is equal to 0.5 mg
RRR-α-tocopherol.
Vitamin E as defined by IOM (
5):
Thus, a 400-IU pill of d-α-tocopherol contains 268 mg 2R-α-tocopherol (400 IU × 0.67 mg/IU), whereas a 400 IU pill of dl-α-tocopherol contains 180 mg 2R-α- tocopherol (400 IU × 0.91 mg/IU ÷ 2).
CHEMISTRY
Antioxidant Activity
Vitamin E functions in vivo as a chain-breaking antioxidant, as reviewed (
15). It is a potent peroxyl radical scavenger and especially protects polyunsaturated fatty acids (PUFAs). When peroxyl radicals (ROO·) are formed, these react 1000 times faster with vitamin E (Vit E-OH) than with PUFA (RH) and form the tocopheroxyl radical (Vit E-O·):
In this way, vitamin E prevents further lipid autooxidation.
The two-electron oxidation product of α-tocopherol is α-tocopheryl quinone. Other α-tocopherol oxidation products that may be formed include 4a,5-epoxy- and 7,8-epoxy-8a (hydroperoxy) tocopherones and their respective hydrolysis products, 2,3-epoxy-α-tocopherol quinone and 5,6-epoxy-α-tocopherol quinone (
16,
17). These products are formed during in vitro oxidation; however, their importance in vivo is unknown. Further oxidation products, including dimers, trimers, and other adducts, have also been described (
18).
Vitamin E Antioxidant Network
The tocopheroxyl radical (Vit E-O·) formed in membranes emerges from the lipid bilayer into the aqueous domain, as reviewed (
19). It is here that the tocopheroxyl radical reacts with vitamin C (or other reductants serving as hydrogen donors, AH), thereby oxidizes the latter, and returns, vitamin E to its reduced state.
Biologically important hydrogen donors include ascorbate (vitamin C) and thiols, especially glutathione. This phenomenon led to the idea of vitamin E recycling in which the antioxidant function of the vitamin E radical is continuously restored by other antioxidants and by the metabolic activity of cells (
20). Regeneration of tocopherol from its radical by vitamin C appears to be a physiologically relevant mechanism, based on studies in humans (see next section), as well as in guinea pigs (
21,
22,
23) and other rodents (
24).
Increased Utilization of Vitamin E by Humans under Oxidative Stress
Oxidative stress caused by ultramarathon running was shown to increase rates of plasma vitamin E disappearance in humans (
25). Moreover, prior vitamin E and C supplementation decreased markers of lipid peroxidation in runners (
26). Chronic oxidative stress and inflammation caused by cigarette smoking also increased α-tocopherol fractional disappearance rates in cigarette smokers compared with nonsmokers (
27). Moreover, the smokers with the lowest plasma ascorbic acid concentrations had the fastest α-tocopherol disappearance rates, presumably because vitamin C regenerates vitamin E (
28). In a subsequent study, Bruno et al (
29) showed that not only was marginal vitamin C status in smokers associated with increased rates of vitamin E disappearance from plasma but also these rates could be normalized by prior vitamin C supplementation. Importantly, both α- and γ-tocopherols were similarly affected by vitamin C status, a finding suggesting that oxidation of the tocopherols is the mechanism for the faster vitamin E disappearance in the presence of low vitamin C status (
29).
Structure-Function Relationships of Vitamin E Forms
Numerous reports have noted health benefits of non-α-tocopherols as anti-inflammatory agents, antioxidants, and antiangiogenic compounds both in atherosclerosis and in cancer protection, as reviewed (
30,
31). One mechanism in which α-tocopherol cannot participate is scavenging reactive nitrogen species. In vitro, γ-, β- or δ-tocopherols can be nitrated (
32,
33,
34). Hoglen et al (
35) demonstrated that 5-nitro-γ-tocopherol (2,7,8-trimethyl-2-[4,8,12-trimethyldecyl]-5-nitro-6-chromanol [NGT]) is the major reactive product between peroxynitrite and γ-tocopherol. NGT has been detected in the plasma of zymosan-treated rats (
36), in the plasma of patients with coronary artery disease (
37) and of cigarette smokers (
38), and in brains collected post-mortem from patients with Alzheimer disease (
39).
DIETARY SOURCES
The richest food sources of vitamin E are almonds, sunflower seeds, and edible vegetable oils (
40), which contain varying proportions of the eight homologs: α-, β -, γ-, and δ-tocopherols or tocotrienols.
RRR-α-tocopherol is especially high in wheat germ oil, safflower oil, and sunflower oil, whereas soybean and corn oils contain predominantly γ-tocopherol, as well as some tocotrienols. Foods that have been fortified with
all-rac-α-tocopheryl acetate include some breakfast cereals, tomato juice, orange juice, and milk.
DIETARY REFERENCE INTAKES
The dietary reference intakes (DRIs) for vitamin C, vitamin E, selenium, and carotenoids were published in 2000 (
5). The DRIs distinguish between
RRR– and
all-rac-α-tocopherol because these structures are physically different and have different fates with respect to transport and metabolism (
5).
The estimated average requirement (EAR) was based on the amount of 2
R-α-tocopherol intake that reversed in vitro peroxide-induced erythrocyte hemolysis in men who were vitamin E deficient as a result of consuming a vitamin E-deficient diet for 5 years, as reviewed (
5). The RDA values for α-tocopherol by life stage are given in
Table 19.1. The EAR of 12 mg 2
R-α-tocopherol was chosen because intakes at this level and above resulted in plasma α-tocopherol concentrations that prevented in vitro hydrogen peroxide-induced erythrocyte hemolysis. The assumption was made that men and women would have similar requirements because women, despite their
lower body weight, have a larger percentage body fat needing antioxidant protection. The RDA for adults (both men and women >19 years old), defined as 2
R-α-tocopherol, is 15 mg/day.
The tolerable upper intake level (UL) was set at 1000 mg/day for vitamin E (any form of supplemental α-tocopherol) (
5). This was one of the few UL that was set using data from rats because sufficient and appropriate quantitative data assessing long-term adverse effects of vitamin E supplements in humans were not available.
The amount of α-tocopherol consumed by most US adults is sufficient to prevent overt symptoms of deficiency (
41). The actual quantities consumed by US adults are closer to 8 mg, however, as assessed by various surveys (
42,
43,
44). Thus, 93% of men and 96% of women in the United States do not consume 12 mg vitamin E daily (
45). The report from the 2010 Dietary Guidelines Committee acknowledged this discrepancy between intake and recommendations but did not promote consumption of most foods containing high amounts of α-tocopherol because these are generally high-fat foods (
45a).
Most people do not consume 15 mg α-tocopherol daily, and therefore they may be at increased risk for various chronic diseases. Previously, the Alpha-Tocopherol Beta-Carotene cancer prevention trial found that daily supplements (vitamin E [50 mg
dl-α-tocopheryl acetate] or β-carotene [20 mg]) for 5 years did not decrease cancer incidence (
46). A subsequent report assessing baseline vitamin E status and dietary intakes described the 29,092 men who had been followed for 19 years since the study’s initiation, during which time 13,380 deaths ensued. The men at baseline in the highest compared with the lowest serum α-tocopherol quintiles had significantly lower risks of total and cause-specific mortality, including cardiovascular disease and cancer (
47). The optimum relative reductions in mortality occurred at serum α-tocopherol concentrations of 13 to 14 mg/L (30 to 32 µmol/L) or higher and were associated with an estimated dietary vitamin E intake of 12 mg α-tocopherol (
47), a dietary value not different from the EAR (12 mg) proposed by the IOM (
5). This finding suggests a health benefit of obtaining the RDA amount of vitamin E from the diet.