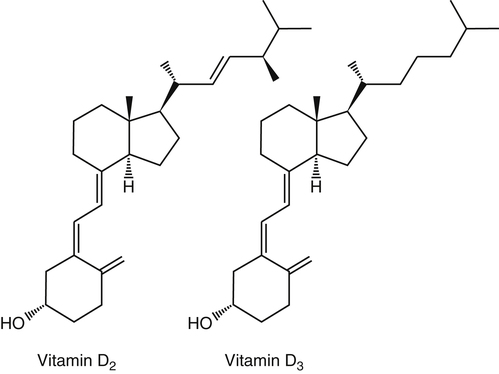
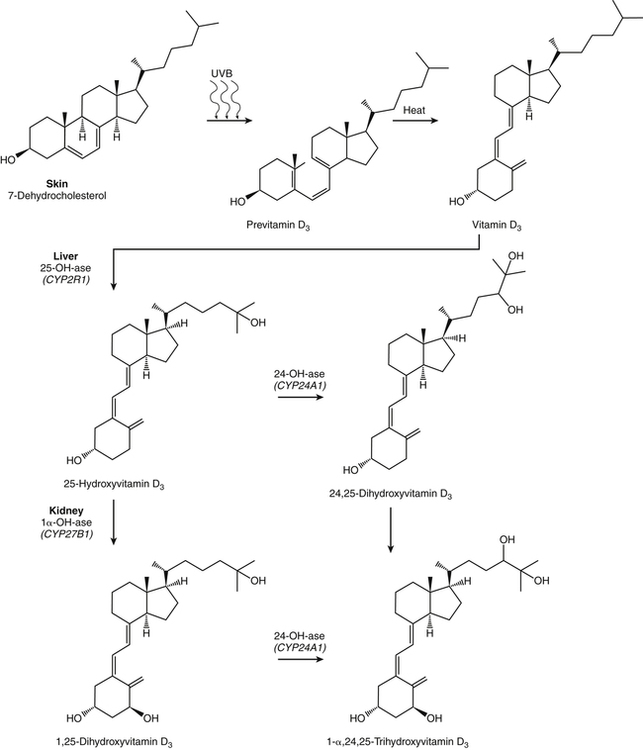
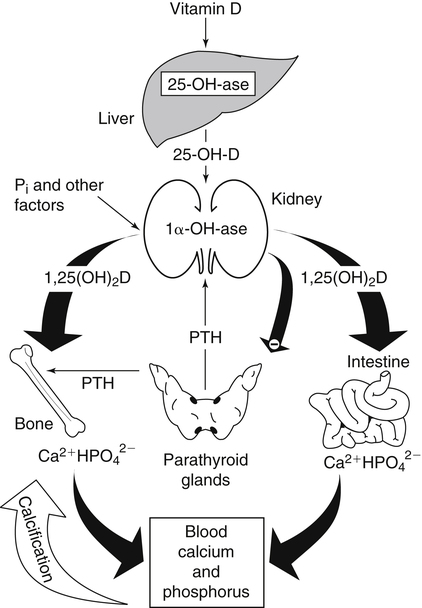
The discovery of vitamin D and its role in promoting bone health in the early decades of the twentieth century contributed to a dramatic decline in the incidence and prevalence of rickets that plagued children in the industrializing nations around the globe. In the years to come, many governments and medical organizations established public health guidelines for adequate intakes of vitamin D and calcium to promote bone health and prevent rickets and adult osteomalacia. In subsequent decades, the greater understanding of vitamin D biology coupled with the rapid growth of epidemiological and clinical studies examining relationships of vitamin D status to various diseases has stimulated an interest in the multifaceted and complex roles of this unique nutrient in an expanding array of human health outcomes. A distinctive aspect of vitamin D is that it can be synthesized by humans through the action of sunlight on exposed skin. Thus an understanding of vitamin D as both a hormone and nutrient presents challenges for the development of Dietary Reference Intake (DRI) values. The rapidly accumulating knowledge in recent years led to a revision of the DRIs for vitamin D and calcium (see Chapter 32) in 2011 by the Institute of Medicine (IOM) that will form the basis for efforts to promote health in the years to come. The terms vitamin D and calciferol refer collectively to the two major nutritionally relevant chemical forms known as vitamin D2 (ergocalciferol) and vitamin D3 (cholecalciferol). Vitamin D3 is the form produced in the skin of vertebrate animals when exposed to ultraviolet (UV) irradiation, whereas vitamin D2 is produced from the precursor ergosterol by some phytoplankton, yeast, invertebrates, and fungi in response to UV irradiation. Vitamin D2 is not synthesized by animals or land plants. Structural differences in the side chains (Figure 31-1) characterize the molecules, yet the biological activity of vitamin D2 and D3 are considered to be essentially equal in regards to correction of vitamin D deficiency. Vitamins D2 and D3 are both commercially synthesized and formulated into dietary supplements and may be added to fortify foods. Studies in animal models suggest that vitamin D2 may have lower toxicity than vitamin D3, but this has yet to be demonstrated in humans. Vitamin D can be synthesized by humans through an elegantly regulated multistep process that begins in the innermost strata of the skin, the stratum basal and stratum spinosum. 7-Dehydrocholesterol (7-DHC) is an immediate precursor of cholesterol synthesis and is abundantly synthesized by the differentiating cells of the epidermis and dermis. Following exposure to sunlight, 7-DHC in the skin absorbs ultraviolet B (UVB) photons with energies between 290 and 320 nm. This process causes a transformation of 7-DHC to previtamin D3 (Tian et al., 1994) (Figure 31-2). Although UV tanning lamps, including those in tanning beds, are dominated by light in the UVA spectrum, up to 10% of total UV exposure may be derived from UVB and impact vitamin D3 synthesis. At normal body temperature, the previtamin D3 undergoes a thermal isomerization of its double bonds to form vitamin D3 (see Figure 31-2). As previtamin D3 is converted to vitamin D3, its three-dimensional structure changes, and this facilitates the translocation of vitamin D3 from the skin cells to the bloodstream. In the circulation, the vitamin D3 produced in the skin is bound to a specific α1-globulin known as vitamin D–binding protein (VDBP), the major plasma carrier of all vitamin D metabolites. VDBP may help sustain cutaneous synthesis by removing the end products and reducing negative feedback regulation. The synthesis of vitamin D3 is impacted by the availability of substrate, the intensity of irradiation reaching the dermis, and the length of exposure, ultimately reaching equilibrium between degradation and synthesis (Holick, 1995a). Indeed, toxic levels of vitamin D accumulating from skin synthesis are not known to occur. If vitamin D3 does not exit from the skin into the circulation before being exposed to additional sunlight, it is degraded to many inactive forms, such as lumisterol and tachysterol (Holick, 1995a). In animals covered with fur or feathers, unique evolutionary adaptations allow vitamin D to be generated within the oily secretions deposited on the fur and feathers and ingested during grooming (Agarwal and Stout, 2004). Vitamin D can also be obtained from the diet as vitamin D2 or vitamin D3. Because vitamin D is fat-soluble, the vitamin is absorbed with other lipids in the intestine, a process facilitated by bile and pancreatic lipase (Holick, 1995b). Upon absorption, vitamin D appears in the chylomicrons that are released by the enterocytes and enters the lymphatic system, which drains into the venous bloodstream. Lipoprotein lipase, particularly in adipose tissue, acts upon chylomicron lipids and may result in a fraction of the vitamin D being taken up by fat cells. This observation suggests a mechanism whereby increased adiposity causes sequestering of vitamin D and is related to lower vitamin D status (IOM, 2011). Indeed, adipose tissue sequestration of vitamin D represents a nonspecific process, and these stores may not be actively used in periods of need (IOM, 2011). Thus obese individuals may require higher intakes of vitamin D to achieve serum concentrations of 25-hydroxyvitamin D (25OHD) comparable to those observed among lean individuals (IOM, 2011). Following depletion of triacylglycerols, the cholesterol-rich chylomicron remnants which still contain a significant fraction of the absorbed vitamin D are subsequently taken up by the liver. Circulating vitamin D2 or vitamin D3 in chylomicron remnants, as well as VDBP-bound vitamin D3 from endogenous synthesis, are taken up by the liver and hydroxylated by hepatic 25-hydroxylase (25-OH-ase), a mitochondrial and microsomal (endoplasmic reticulum) enzyme likely encoded by the gene CYP2R1 (cytochrome P450 2R1) to form 25-hydroxycholecalciferol (25OHD) (see Figure 31-2). This enzyme is efficient and exhibits little or no feedback regulation. Like vitamin D, 25OHD circulates in plasma as a complex with VDBP. The plasma 25OHD level is currently considered to be the best measure of vitamin D status. The subsequent metabolism of 25OHD to 1,25-dihydroxyvitamin D (1,25[OH]2D) is the critical step in the endocrine regulatory process that is essential for maintaining homeostatic regulation of plasma calcium and phosphorus concentrations in ranges compatible with life and promotion of bone health. Delivery of 25OHD to the kidneys, and thus its conversion to 1,25(OH)2D, depends upon the glomerular filtration of 25OHD–VDBP complexes from the plasma, followed by megalin-mediated endocytic uptake of 25OHD–VDBP from the glomerular filtrate into the proximal tubular cells of the kidney (Leheste et al., 2003). VDBP is a ligand of cubilin and megalin, which are two membrane-associated proteins that act in concert to mediate endocytic uptake of various small proteins and vitamin-carrier complexes across eptihelia. Therefore these endocytic receptors in the renal tubules are the major means by which 25OHD is targeted to the kidney (Nykjaer et al., 1999). The enzymatic reaction producing 1,25(OH)2D is highly regulated and is catalyzed by the action of mitochondrial 25-hydroxyvitamin D 1α-hydroxylase (1α-OH-ase), encoded by the CYP27B1 (cytochrome P450 27B) gene. The expression of CYP27B1 is stimulated by parathyroid hormone (PTH), whose main function is to respond to declining plasma calcium concentrations and act upon bone to stimulate calcium release, enhance active reabsorption of calcium from the kidney distal tubules, and stimulate production of 1,25(OH)2D, which in turn will increase the absorption of calcium by the intestine (Figure 31-3). As plasma concentrations of 1,25(OH)2D increase, a negative feedback loop suppresses CYP27B1 expression. Recently, the phosphaturic hormone, fibroblast-like growth factor-23, has been implicated as a counterregulatory hormone involved in suppressing CYP27B1 expression (Galitzer et al., 2008; Bergwitz and Juppner, 2010; see Chapter 32, Figure 32-3). 1,25(OH)2D is the high affinity ligand for the vitamin D receptor (VDR; see next section) and is transported from the kidney to target tissues via the VDBP. Studies in transgenic mice lacking VDBP gene expression show only 10% to 15% of the plasma vitamin D when compared to controls. Other differences include a shortened half-life of plasma vitamin D, increased urinary concentrations of vitamin D, and a more rapid uptake of vitamin D by the liver that is associated with an increased catabolism to degradation products and their excretion primarily in the bile and feces (Jones et al., 1998; Berg, 1999). Catabolism of calciferol to more polar and readily excretable inactive metabolites, 24,25(OH)2D and 1α,24,25(OH)2D, begins with the 24-hydroxylation of either 25OHD or 1,25(OH)2D, respectively. This hydroxylation reaction is catalyzed by the cytochrome P450 enzyme, 25-hydroxyvitamin D 24-hydroxylase (24-OH-ase), a protein encoded by the CYP24A1 gene (Jones et al., 1998). Although CYP24A1 is highly expressed in the kidney tubule, its tissue distribution is quite broad including the intestine, osteoblasts, placenta, keratinocytes, and prostate. The catabolic CYP24A1 gene is potently induced by high plasma concentrations of 1,25(OH)2D. Studies with mice lacking CYP24A1 demonstrate an inability to clear plasma vitamin D, leading to a near 50% lethality at weaning (Masuda et al., 2005). 1,25(OH)2D is considered to be the biologically active hormonal form of vitamin D that is responsible for carrying out most, if not all, of the biological functions of vitamin D. A major function of 1,25(OH)2D is to help maintain plasma concentrations of calcium within a normal range, which is achieved in part by increasing the efficiency of intestinal calcium absorption (Fleet and Schoch, 2010). 1,25(OH)2D can dramatically impact the efficiency of intestinal calcium absorption from a basal level of 10% to 15% up to a level of 30% to 80%. 1,25(OH)2D also increases the efficiency of intestinal phosphorus absorption. Other key tissues affected by 1,25(OH)2D are the bones and kidneys. 1,25(OH)2D along with PTH induces the formation and activation of osteoclasts, which function in the mobilization of calcium from bone (see Chapter 32, Figure 32-4). In addition, mature osteoblasts respond to 1,25(OH)2D by increasing their expression of proteins involved in bone remodeling. 1,25(OH)2D, together with PTH, also stimulates the renal distal tubule reabsorption of calcium. Thus overall, 1,25(OH)2D acts on the intestines, bones, and kidneys to elevate plasma calcium (see Figure 31-3), which is required for mineralization of bone and for many other biological actions (see Chapter 32). Most of the effects of 1,25(OH)2D on calcium homeostasis are mediated through alterations in gene expression. The identification of the vitamin D receptor (VDR) in the 1970s, coupled with the elucidation of the vitamin D metabolic pathways described previously, allowed investigators to pursue the fundamental mechanisms whereby vitamin D promotes bone health and regulates various functions in a growing array of tissues. As a member of the steroid hormone receptor superfamily, the VDR has many characteristics similar to receptors for thyroid hormone, estrogen, testosterone, and retinoids. Localized primarily in the nuclei of target cells, the VDR has a high affinity for 1,25(OH)2D hormone (or calcitriol). Binding of calcitriol to VDR causes it to be phosphorylated and then recruited by one of the three 9-cis-retinoid X receptors (RXRα, RXRβ, and RXRγ). These heterodimers interact with coregulatory proteins, allowing the complex to bind to specific genomic sequences in the promoter region of genes. The VDR-specific binding sites are named vitamin D–responsive elements (VDREs) (Figure 31-4). The VDR and 1,25(OH)2D ligand were found in the predicted target tissues of the intestine, bone, and kidney and strongly linked to the expected biological responses of increasing duodenal calcium absorption and enhanced bone mineralization. The best-characterized gene products that are induced by 1,25(OH)2D in osteoblasts are osteocalcin, osteopontin, and alkaline phosphatase. Collectively, these proteins have a central role in the bone remodeling process. The major gene products induced in the small intestine by 1,25(OH)2D are the epithelial calcium channel protein (CaT1) which directly affects the entry of calcium into the intestinal absorptive cell as well as several proteins that alter the flux of calcium across the intestinal absorptive cell (i.e., calcium binding protein [CaBP]) (see Figure 31-4). The translation of these laboratory findings to clinical application was rapid, with the demonstration that renal failure patients with poor synthesis of 1,25(OH)2D responded to the pharmacological administration of the active hormone with enhanced calcium absorption and improved bone health (Brickman et al., 1972). In recent decades, investigators have reported that the VDR is present in many tissues (Norman and Bouillon, 2010), most of which are not involved in calcium and phosphate homeostasis. The knowledge that the VDR acts as a transcription factor coupled with the presence of VDREs in the promoter regions of over 3% of genes in the human genome has greatly expanded research efforts to better elucidate the roles of vitamin D in the tissues and organs where the receptor is expressed. VDREs, considered the hallmark for vitamin D regulation of gene expression, are present in genes involved in a diverse range of processes, including the regulation of cell proliferation, differentiation, and apoptosis. In parallel, this knowledge also provides a molecular foundation for the study of an expanding array of disease processes that may theoretically be impacted by alterations in vitamin D status. Adding to the complexity of the vitamin D regulatory network, we now recognize that 1α-OH-ase is present in several tissues in addition to the kidney and that the local synthesis of the active hormone may have an autocrine or paracrine action to fine-tune tissue responses to vitamin D. An emerging area of research focuses upon rapid steroid actions that mediate cellular effects occurring over seconds and minutes, as compared to the well-established genomic action of steroid hormones and receptor-mediated genomic actions that occur over hours or days and can be blocked by inhibitors of transcription (Mizwicki and Norman, 2009). Investigators have reported evidence for nongenomic actions of 1,25(OH)2D that are independent of receptor-mediated transcription in a variety of cell types and tissues (Losel and Wehling, 2003; Norman, 2006; Mizwicki and Norman, 2009; Fleet and Schoch, 2010). Among the strongest examples for nongenomic actions of 1,25(OH)2D is the induction of rapid intestinal absorption of calcium, called transcaltachia (Nemere et al., 1984; Huhtakangas et al., 2004; Fleet and Schoch, 2010), a process mediated by interactions with a membrane-associated, rapid response, steroid-binding protein (Nemere et al., 2004). Transcaltachia may lead to activation of multiple signaling cascades, such as phosphorylation of protein kinase C, the opening of cellular calcium channels resulting in an increase in intracellular calcium uptake, and further activation of the mitogen activiated protein kinase pathways. These cascades may enhance expression of the VDR, providing a basis for cross-talk and cooperation with the genomic pathway to induce biological responses. Among scientists and the medical community, it is generally agreed that the serum concentration of 25OHD is the best surrogate biomarker of vitamin D status. Because the hepatic vitamin D 25-OH-ase is efficient and not tightly regulated, any increase in vitamin D intake or cutaneous production of vitamin D3 leads to an increase in the circulating concentration of 25OHD. Hence, 25OHD is a valuable marker for determining an individual’s vitamin D status at a specific time. Low or undetectable circulating concentrations of 25OHD (less than 10 ng/mL) are diagnostic of vitamin D deficiency. Currently, several different types of assays are employed, each with their own strengths and weaknesses (Carter et al., 2010). Antibody-based and liquid chromatography–based methods are currently used and are equivalent in terms of measuring total 25OHD. However, controversy remains over the performance of these assays in various clinical and research laboratories (IOM, 2011). For example, assay shift and drift must be considered in the evaluation of data from the NHANES (National Health and Nutrition Examination Survey) reports (IOM, 2011). Serum vitamin D is not a biomarker for dietary intake as the relationship between dietary intake and circulating 25OHD is nonlinear. Many factors may impact the association between vitamin D intake and serum 25OHD; the most critical is endogenous synthesis (Cashman et al., 2008a, 2008b, 2009; Smith et al., 2009; IOM, 2011). Evidence suggests that increasing the serum level of 25OHD above 50 nmol/L requires more vitamin D intake than does increasing serum 25OHD levels when initial concentrations are lower (Aloia et al., 2008). A crude estimate is that for each additional 1,000 IU/day of vitamin D intake, the serum 25OHD concentration may increase by 10 to 20 nmol/L. A key area of controversy relates to defining specific cutoff points of circulating 25OHD levels to define deficiency, marginal deficiency, normal ranges, and concentrations associated with toxicity. The recent National Academy of Sciences committee recognized the controversy regarding this issue, and many committee members suggested the need to develop a consensus that impacts the interpretation of clinical testing and the medical management of individuals, in addition to helping scientists and public health officials interpret human population-based studies (Ross et al., 2011). It is also important to consider the need for studies to define serum concentration standards in different age groups, from infants to the elderly, and to consider potential ethnic and gender differences. Finally, it is critical to develop studies to assess the combined and individual contributions of solar radiation and dietary intake to serum concentrations of 25OHD in healthy and diseased populations, in terms of mean values and the many genetic and environmental factors that may also impact vitamin D status. In spite of the aforementioned limitations and concerns, a general strategy for interpretation of serum concentrations of 25OHD can be stated. Those with serum concentrations less than 5 ng/mL (12 nmol/L) are considered to be at risk of severe vitamin D deficiency (IOM, 2011). Individuals with a serum concentration in this range for 1 to 2 years may develop clinically relevant rickets or osteomalacia, particularly if dietary calcium intake is marginal (IOM, 2011). A serum concentration of 20 ng/mL (50 nmol/L) of 25OHD is considered adequate to satisfy the needs for bone health in the vast majority (97.5%) of healthy adults. The range of optimal serum 25OHD concentrations for other health outcomes related to vitamin D (such as cancer prevention or the prevention of frailty or falls in the elderly) is the subject of much debate. There is clearly a wide range of serum 25OHD concentrations where no clear evidence of toxicity has been reported. Many have assumed that the physiological production of vitamin D in humans with extensive exposure to the sun should provide information about levels of 25OHD that are not toxic. In studies of outdoor workers, such as lifeguards, mean serum concentrations of 25OHD of nearly 125 nmol/L (50 ng/mL) are observed (IOM, 2011). There are a few well-documented human studies of vitamin D toxicity in which serum 25OHD was measured (IOM, 2011). Based on these case-reports of vitamin D intoxication, serum concentrations above 100 to 150 ng/mL (250 to 375 nmol/L) raise concern for toxicity (IOM, 2011). Total vitamin D intake includes both foods and dietary supplements. Foods naturally containing vitamin D are limited (U.S. Department of Agriculture [USDA], Agricultural Research Service [ARS], 2010). The richest sources of dietary vitamin D are fatty fish, such as salmon and sardines; liver oil extracts, such as cod liver oil; and egg yolks. In the United States, fluid milk is voluntarily fortified with 400 IU per quart (385 IU/L), and similar quantities are usually added to fortified plant-based milk substitutes. More recently, some cereals and breads have been fortified with vitamin D. Other milk-based products, including ice cream and cheeses, are not typically fortified with vitamin D, whereas some yogurts are fortified. In Canada, fortification of fluid milk (350 to 450 IU/L) and margarine (530 IU/g) is mandatory. Infant formulas are also
Vitamin D
Dietary and Endogenous Sources of Vitamin D
Endogenous Synthesis and Metabolism of Vitamin D
Uptake of Dietary Vitamin D
Metabolism of Vitamin D in Liver
Metabolism of Vitamin D in Kidney
Catabolism of Vitamin D
Biological Actions of Vitamin D
Genomic Actions of Vitamin D
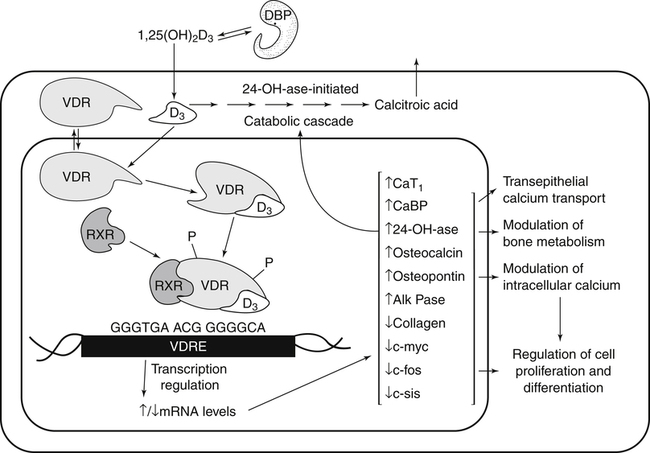
Nongenomic Actions of Vitamin D
Evaluation of Vitamin D Status
Dietary Sources of Vitamin D
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Basicmedical Key
Fastest Basicmedical Insight Engine