TERMINOLOGY, CHEMISTRY, METABOLIC ROLES, INTERACTIONS WITH OTHER COMPOUNDS, AND BASIC IMPORTANCE IN NORMAL FUNCTIONS
Terminology and Chemical Properties: Formation, Oxidation-Reduction, and Degradation
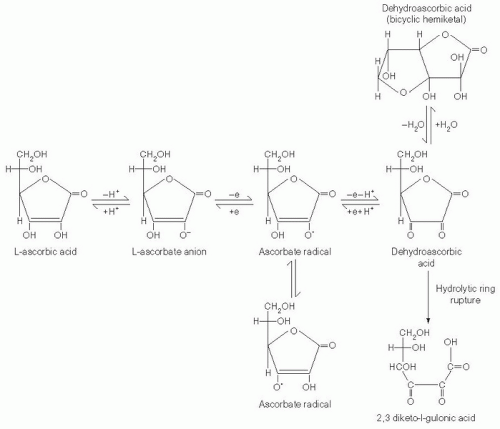
Vitamin C (L-ascorbic acid, ascorbate), a water-soluble micronutrient essential for humans, is a six-carbon α-ketolactone weak acid with a pH of 4.2 and a molecular
weight of 176 (
Fig. 29.1). Plants use glucose and fructose to synthesize vitamin C. Vitamin C is abundant in plant leaves and in chloroplasts, and it may have a role in photosynthesis, stress resistance, plant growth, and development. Most mammals synthesize vitamin C from glucose in the liver, whereas some birds, reptiles, and amphibians synthesize the vitamin in the kidney (
5). Vitamin C is not synthesized by humans and nonhuman primates because of their lack of gulonolactone oxidase, the terminal enzyme in the biosynthetic pathway of vitamin C from glucose. The gulonolactose oxidase gene became nonfunctional in a common primate ancestor (
5). Guinea pigs, capybaras, bats, and some fish also do not synthesize ascorbate (
6). For all species unable to synthesize ascorbate, it is a vitamin by definition and must be obtained exogenously. Animals unable to synthesize vitamin C usually obtain sufficient amounts from plant diets, but they develop scurvy in captivity without adequate dietary supplementation (
7).
Vitamin C is an electron donor, or reducing agent (see
Fig. 29.1), and all its known functions are attributable to this property. Vitamin C sequentially donates two electrons from the double bond between carbons two and three. When these electrons are lost, vitamin C is oxidized, and another compound is reduced, thereby forestalling oxidation of the reduced compound. Vitamin C is therefore commonly known as an antioxidant.
With loss of the first electron, vitamin C oxidizes to the ascorbate free radical (semidehydroascorbic acid). In comparison with other free radicals, the ascorbate radical is relatively stable and unreactive. Reactive free radicals are reduced by vitamin C, and the less reactive ascorbate radical is formed in their place. This is the basis for characterizing vitamin C as a good free radical scavenger, or antioxidant (
8). Because of the short half-lives (i.e., <10
-3 seconds) of most free radicals, they cannot be measured directly and instead are measured indirectly by
using other agents that form radical species with longer half-lives. The half-life of the ascorbate radical is long enough to be measured directly by electron paramagnetic resonance, however. Ascorbate radical half-life depends on concentration, the presence of trace metals, and oxygen, and it can vary from 10
-3 seconds to minutes.
After formation, the ascorbate radical is either reversibly reduced to vitamin C or loses a second electron and is thereby oxidized to dehydroascorbic acid (
8). Although this substance is more stable than the ascorbate radical, the stability of dehydroascorbic acid depends on its concentration, temperature, and pH, and it is often stable for only minutes (
9). Dehydroascorbic acid may exist in one of several different structural forms (see
Fig. 29.1). Its dominant form in vivo is uncertain, but a good candidate is the hydrated hemiketal (
10). Because dehydroascorbic acid is probably not an acid in vivo, the designation “dehydroascorbate” is incorrect. Formation of both the ascorbate radical and dehydroascorbic acid from vitamin C in biologic systems is mediated by oxidants such as molecular oxygen with or without trace metals (iron and copper), superoxide, hydroxyl radical, hypochlorous acid, and reactive nitrogen species.
In biologic systems, dehydroascorbic acid has two fates. One is to become hydrolyzed, with irreversible rupture of the ring to yield 2,3-diketogulonic acid. Although 2,3-diketogulonic acid metabolism is not well characterized, its metabolic products probably include oxalate, threonate, xylose, xylonic acid, and lynxonic acid (
9). Carbons from vitamin C were reported to be expired as carbon dioxide in animals, but this probably does not occur in humans (
11). Of vitamin C metabolites formed by dehydroascorbic acid hydrolysis, oxalate is an end product with clinical significance (see the section “Manifestations of Vitamin C Deficiency and Excess”).
The second fate of dehydroascorbic acid is to become reduced, either to the ascorbate radical by addition of one electron, or to vitamin C by addition of two electrons. Dehydroascorbic acid reduction in biologic tissues occurs chemically or is protein dependent (
5). Chemical reduction of dehydroascorbic acid is mediated in vivo by glutathione, with formation of glutathione disulfide. Enzymatic reduction of dehydroascorbic acid in vivo, with an electron donor, is often faster than by chemical reduction alone. Reduced nicotinamide adenine dinucleotide phosphate-dependent regenerating enzymes include 3-α-hydroxysteroid dehydrogenase and thioredoxin reductase. Glutathione-dependent regenerating enzymes include glutaredoxin (thioltransferase), protein disulfide isomerase, and dehydroascorbate (sic) reductase, with Michaelis-Menten constants (K
ms) for dehydroascorbic acid of 250 µM to several millimolars. Protein (enzyme)-mediated reduction results in ascorbate formation without ascorbate radical as an intermediate, as described for glutaredoxin.
Ascorbate radical can also be reduced to vitamin C. Although the reducing activities responsible have not been purified, several reducing activities have been reported in membranes of mitochondria, microsomes, and erythrocytes. The cytosolic enzyme thioredoxin reductase also reduces the ascorbate radical (
5).
In humans, ascorbate radical and dehydroascorbic acid reduction efficiency is incomplete. When vitamin C is removed from diets of healthy humans, deficiency occurs by 30 days, even if the subjects were initially saturated with vitamin C (
12,
13) (see the discussion about pharmacokinetics under the section “Physiology”). These data are a summed measure of both oxidation and reduction rates. The overall direction is vitamin C utilization, in which ascorbic acid is oxidized to dehydroascorbic acid, and dehydroascorbic acid undergoes irreversible hydrolysis.