Viral Hepatitis
KEY CONCEPTS
![]() Hepatitis A is transmitted via the fecal–oral route. Transmission is most likely to occur through travel to countries with high rates of hepatitis A, poor sanitation and hygiene, and overcrowded areas.
Hepatitis A is transmitted via the fecal–oral route. Transmission is most likely to occur through travel to countries with high rates of hepatitis A, poor sanitation and hygiene, and overcrowded areas.
![]() Hepatitis A causes an acute, self-limiting illness and does not lead to chronic infection. There are three stages of infection: incubation, acute hepatitis, and convalescence. Rarely, the infection progresses to liver failure.
Hepatitis A causes an acute, self-limiting illness and does not lead to chronic infection. There are three stages of infection: incubation, acute hepatitis, and convalescence. Rarely, the infection progresses to liver failure.
![]() Treatment of hepatitis A consists of supportive care. There is no role for antiviral agents in treatment.
Treatment of hepatitis A consists of supportive care. There is no role for antiviral agents in treatment.
![]() Hepatitis B causes both acute and chronic infection. Infants and children are at high risk for chronic infection.
Hepatitis B causes both acute and chronic infection. Infants and children are at high risk for chronic infection.
![]() Several therapies are available for hepatitis B, including lamivudine, interferon-alfa, pegylated interferon-alfa, entecavir, adefovir, telbivudine, and tenofovir. Patient status, extent of disease, viral load, and viral resistance are all considered when deciding on treatment.
Several therapies are available for hepatitis B, including lamivudine, interferon-alfa, pegylated interferon-alfa, entecavir, adefovir, telbivudine, and tenofovir. Patient status, extent of disease, viral load, and viral resistance are all considered when deciding on treatment.
![]() Chronic hepatitis B patients may require long-term therapy. Long-term therapy poses a challenge because of the potential for developing resistance. Resistance to lamivudine and telbivudine is most common, limiting the use of these treatments. Optimal treatment of resistant strains is unknown.
Chronic hepatitis B patients may require long-term therapy. Long-term therapy poses a challenge because of the potential for developing resistance. Resistance to lamivudine and telbivudine is most common, limiting the use of these treatments. Optimal treatment of resistant strains is unknown.
![]() Prevention of hepatitis B infections focuses on immunization of all children and at-risk adults.
Prevention of hepatitis B infections focuses on immunization of all children and at-risk adults.
![]() Hepatitis C is an insidious, blood-borne infection. Many people are unaware of their infection and risk significant morbidity and mortality.
Hepatitis C is an insidious, blood-borne infection. Many people are unaware of their infection and risk significant morbidity and mortality.
![]() Combination pegylated interferon and ribavirin therapy with either boceprevir or telaprevir is the treatment of choice for hepatitis C genotype 1 infections. Treatment duration varies depending on response, previous treatment history, and the presence of cirrhosis. For genotypes 2, 3, and 4 the treatment of choice includes pegylated interferon and ribavirin.
Combination pegylated interferon and ribavirin therapy with either boceprevir or telaprevir is the treatment of choice for hepatitis C genotype 1 infections. Treatment duration varies depending on response, previous treatment history, and the presence of cirrhosis. For genotypes 2, 3, and 4 the treatment of choice includes pegylated interferon and ribavirin.
![]() Boceprevir and telaprevir offer significant improvements in outcome for the treatment of hepatitis C genotype 1 infections but pose additional challenges and new concerns for multiple drug interactions.
Boceprevir and telaprevir offer significant improvements in outcome for the treatment of hepatitis C genotype 1 infections but pose additional challenges and new concerns for multiple drug interactions.
The major hepatotrophic viruses responsible for viral hepatitis are hepatitis A, hepatitis B, hepatitis C, delta hepatitis, and hepatitis E. All share clinical, biochemical, immunoserologic, and histologic findings. Both hepatitides A and E are spread through fecal–oral contamination, whereas hepatitides B, C, and delta are transmitted parenterally. Infection with delta hepatitis requires coinfection with hepatitis B. Although the rates of acute infection have declined, viral hepatitis remains a major cause of morbidity and mortality with a significant impact on healthcare costs in the United States. Compared with human immunodeficiency virus (HIV), there are three to five times as many people infected with chronic viral hepatitis. In the United States, there is a general lack of knowledge among healthcare providers, social service providers, and the public regarding the risks of chronic hepatitis B and C infections.1
Unprecedented therapeutic advances have occurred with the treatment for hepatitis C with the approval of new agents, updated guidelines for care, and more novel therapies eagerly anticipated. For both hepatitides B and C, the challenge remains to increase awareness of the viral hepatic epidemic and to prevent the profound morbidity and mortality associated with chronic infection. This chapter focuses on hepatitides A, B, and C.
HEPATITIS A
Hepatitis A virus (HAV), or infectious hepatitis, is often a self-limiting and acute viral infection of the liver posing a health risk worldwide. The infection is rarely fatal. According to the Centers for Disease Control and Prevention (CDC), rates of reported cases of acute clinical hepatitis A infection in the United States continue to decline with 1,670 cases in 2010.2 The significant declines in rates of acute HAV are associated with major vaccination campaigns that successfully reduced the incidence rate.
Epidemiology
Various patient groups are at increased risk for infection with HAV. Children pose a particular problem with the spread of the disease because they often remain clinically asymptomatic and are infectious for longer periods of time than adults. Traditionally, the most likely patient group affected is household or close personal contacts of an infected person. ![]() Infection primarily occurs through the fecal–oral route, by person-to-person, or by ingestion of contaminated food or water. Incidentally, HAV’s prevalence is linked to regions with low socioeconomic status and specifically to those with poor sanitary conditions and overcrowding. International travel and immigration also mitigate potential exposure to the virus.
Infection primarily occurs through the fecal–oral route, by person-to-person, or by ingestion of contaminated food or water. Incidentally, HAV’s prevalence is linked to regions with low socioeconomic status and specifically to those with poor sanitary conditions and overcrowding. International travel and immigration also mitigate potential exposure to the virus.
International travel, in particular travel to HAV endemic areas, continues to be a major risk factor for HAV infection. Other identified risk factors include sexual and household contact with an HAV-infected person, men who have sex with men (MSM), and injection-drug users (IDUs).2 Additional patient groups that are at risk include patients with chronic liver disease and persons working with nonhuman primates. In 2010, 75% of case reports of acute HAV reported no identifiable risk factor.2 Among MSMs, specific sexual practices may be associated with an increased risk for infection.3 Foodborne outbreaks also occur. In general, mortality rates are low but highest among persons ≥75 years of age.2
Despite low endemic rates and successful vaccination programs in the United States, travel to HAV endemic areas is a recognized risk for acquiring acute HAV infections. According to the CDC, the majority of travel-related cases correspond to travel to Central and South America and Mexico.2 Most Americans traveling to Mexico do not consider that country to be a risk in part because of Mexico’s proximity to the United States. Moreover, most tourists falsely believe that higher-end resorts imply safety and that short visits to foreign countries are not associated with a risk for infection. Travel related to international adoptions can also be of risk. In 2009, HAV vaccination was recommended for household members and close personal contacts of newly adopted children from countries of high or intermediate HAV endemicity.4
Etiology
Hepatitis A is a RNA virus belonging to the genus Hepatovirus of the Picornaviridae family. Humans are the only known reservoir for the virus and transmission occurs primarily through the fecal–oral route.6 The virus is stable in the environment for at least a month and requires heating foods to a minimum of 85°C (185°F) for 1 minute or disinfecting with a 1:100 dilution of sodium hypochlorite (bleach) in tap water for inactivation.5,7
Multiple genotypes of the virus exist and although the clinical implications of infection by particular type are unknown, types I and III are the most commonly identified in human outbreaks.6
Pathophysiology
HAV infection is usually acute, self-limiting, and confers lifelong immunity. HAV’s life cycle in the human host classically begins with ingestion of the virus. Absorption in the stomach or small intestine allows entry into the circulation and uptake by the liver. Replication of the virus occurs within hepatocytes and GI epithelial cells. New virus particles are released into the blood and secreted into bile by the liver. The virus is then either reabsorbed to continue its cycle or excreted in the stool. The enterohepatic cycle will continue until interrupted by antibody neutralization.6 The exact mechanism of replication and secretion is unknown; however, the initial viral expansion does not seem to be associated with hepatic injury as peak viral fecal excretion precedes clinical signs and symptoms of infection.5
Clinical Presentation
![]() The incubation period of HAV is approximately 28 days, with a range of 15 to 50 days. Viremia occurs within 1 to 2 weeks of exposure as patients begin to shed the virus.5 Table 26-1 summarizes the clinical features of acute hepatitis A. Peak fecal shedding of the virus precedes the onset of clinical symptoms and elevated liver enzymes. Acute hepatitis follows, beginning with the preicteric or prodromal period. The phase is marked by an abrupt onset of nonspecific symptoms, some very mild.5 Other, more unusual symptoms include chills, myalgia, arthralgia, cough, constipation, diarrhea, pruritus, and urticaria. The phase generally lasts 2 months. There are no specific symptoms unique to HAV. Liver enzyme levels rise within the first weeks of infection, peaking approximately in the fourth week and normalizing by the eighth week. Conjugated bilirubinemia, clinically evident as dark urine, precedes the onset of the icteric period. GI symptoms may persist or subside during this time and some patients may have hepatomegaly. Duration of the icteric period varies and corresponds to disease duration. It averages between 7 and 30 days.6
The incubation period of HAV is approximately 28 days, with a range of 15 to 50 days. Viremia occurs within 1 to 2 weeks of exposure as patients begin to shed the virus.5 Table 26-1 summarizes the clinical features of acute hepatitis A. Peak fecal shedding of the virus precedes the onset of clinical symptoms and elevated liver enzymes. Acute hepatitis follows, beginning with the preicteric or prodromal period. The phase is marked by an abrupt onset of nonspecific symptoms, some very mild.5 Other, more unusual symptoms include chills, myalgia, arthralgia, cough, constipation, diarrhea, pruritus, and urticaria. The phase generally lasts 2 months. There are no specific symptoms unique to HAV. Liver enzyme levels rise within the first weeks of infection, peaking approximately in the fourth week and normalizing by the eighth week. Conjugated bilirubinemia, clinically evident as dark urine, precedes the onset of the icteric period. GI symptoms may persist or subside during this time and some patients may have hepatomegaly. Duration of the icteric period varies and corresponds to disease duration. It averages between 7 and 30 days.6
TABLE 26-1 Clinical Presentation of Acute Hepatitis A
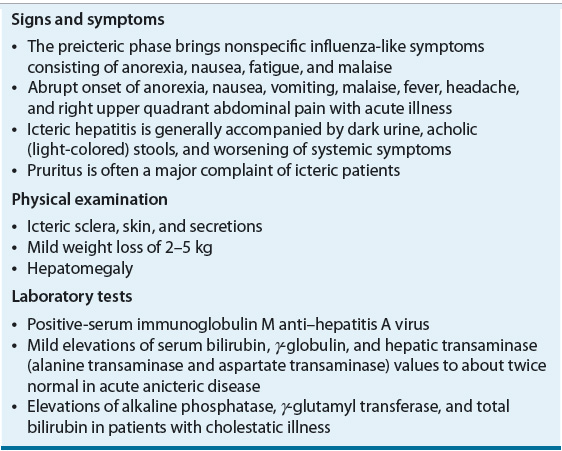
Symptoms and severity of HAV vary according to age. Children younger than 6 years of age typically are asymptomatic. Symptoms, if they do occur, do not include jaundice. In older children and adults, the majority of patients present with symptoms that last less than 2 months and 70% of adults experience jaundice. Peak viral shedding precedes the onset of GI symptoms in adults. In young children, shedding can occur for months following diagnosis.5 Because children are often asymptomatic and will shed the virus for long periods of time, they can serve as a reservoir for the spread of HAV.
Serum HAV RNA is detectable approximately 2 weeks prior to the onset of symptoms or peak alanine aminotransferase (ALT) levels and can persist for an average of 79 days after the onset of symptoms. In some patients, serum HAV is detectable for more than a year.11 The use of nucleic acid sequencing to detect HAV RNA is limited to research and instead immunoglobulin (Ig) M antibody to HAV (anti-HAV) is required for a diagnosis of acute infection in clinical settings. IgM anti-HAV is detectable 5 to 10 days prior to symptomatic HAV infections in the majority of patients. IgG anti-HAV replaces IgM and indicates host immunity following the acute phase of the infection.7 FDA-approved assays for serologic testing detect IgM anti-HAV only and total anti-HAV (IgM and IgG anti-HAV). Patients who have detectable total anti-HAV with a negative IgM have resolved their infection. Patients who are successfully immunized or who receive Ig may have lower levels of total anti-HAV that are below the levels of detection of most commercial assays.5,7 Concentrations of antibody often fall to 10 to 100 times lower than what would be expected after a natural course of infection. Although a positive anti-HAV result confirms protection, undetectable concentration of anti-HAV may not necessarily imply that protective levels were not achieved.7
HAV does not lead to chronic infections. Some patients may experience symptoms for up to 9 months. Rarely, patients experience complications from HAV, including relapsing hepatitis, cholestatic hepatitis, and fulminant hepatitis. Fatalities from HAV are generally rare, although more likely in patients older than age 50 years and in persons with preexisting liver disease.7
A diagnosis of HAV is based on clinical criteria of an acute onset of fatigue, abdominal pain, loss of appetite, intermittent nausea and vomiting, jaundice or elevated serum aminotransferase levels, and serologic testing for IgM anti-HAV. Serologic testing is necessary to differentiate the diagnosis from other types of hepatitis.
TREATMENT
Desired Outcome
![]() The majority of people infected with HAV can be expected to fully recover without clinical sequelae.6 Nearly all individuals will have clinical resolution within 6 months of the infection, and a majority will have done so by 2 months. Rarely, symptoms persist for longer or patients relapse. The ultimate goal of therapy is complete clinical resolution. Other goals include reducing complications from the infection, normalization of liver function, and reducing infectivity and transmission. Prevention of HAV infection is important because significant costs are accrued during acute HAV infections, from both direct costs of hospitalizations and indirect costs from loss of work days.
The majority of people infected with HAV can be expected to fully recover without clinical sequelae.6 Nearly all individuals will have clinical resolution within 6 months of the infection, and a majority will have done so by 2 months. Rarely, symptoms persist for longer or patients relapse. The ultimate goal of therapy is complete clinical resolution. Other goals include reducing complications from the infection, normalization of liver function, and reducing infectivity and transmission. Prevention of HAV infection is important because significant costs are accrued during acute HAV infections, from both direct costs of hospitalizations and indirect costs from loss of work days.
General Approach to Treatment
No specific treatment options exist for HAV infections. Instead, patients should receive general supportive care. Prevention and prophylaxis are key to managing the virus. The importance of good hand hygiene cannot be overemphasized in preventing disease transmission. Ig is used for preexposure and postexposure prophylaxis, and offers passive immunity. Active immunity is achieved through vaccination. Vaccines were approved for use in 1995 and implemented in the routine vaccination of children, as well as at-risk adults, to reduce the overall incidence of HAV.7
Prevaccination serologic testing to determine susceptibility is generally not recommended. In some cases, testing may be cost-effective if the cost of the test is less than that of the vaccine and if the person is from a moderate to high endemic area and likely to have prior immunity. Prevaccination serologic testing of children is not recommended. Similarly, because of high vaccine response, postvaccine serologic testing is not recommended.7
Prevention of Hepatitis A
HAV is easily preventable with vaccination. Because children often serve as reservoirs of the disease, vaccine programs have targeted children as the most effective means to control HAV. Two vaccines for HAV are available and are incorporated into the routine childhood vaccination schedule. In October 2005, the FDA reduced the minimum age for the vaccines to 12 months of age. In response, the Advisory Committee on Immunization Practices (ACIP) recommended expanding vaccine coverage to all children, including catch-up programs for children living in areas without existing vaccination programs. The new recommendations were enacted in the attempt to further reduce HAV incidence rates and possibly to eradicate the virus.13 Following a CDC health advisory report of HAV infection from international adoptees, in 2009 ACIP updated its guidelines to include hepatitis A vaccination for previously unvaccinated persons anticipating close personal contact with international adoptees from a country of high or intermediate endemicity. Complete HAV vaccination recommendations are available from the CDC (Table 26-2).
TABLE 26-2 Recommendations for Hepatitis A Virus (HAV) Vaccination

Routine prevention of HAV transmission includes regular hand washing with soap and water after using the bathroom, changing a diaper, and before food preparation. For travelers to countries with high endemic rates of HAV, even short-term stays in urban and upscale resorts are not risk-free.7 In particular, contaminated water and ice, fresh produce, and any uncooked foods pose a risk.6
Vaccines to Prevent Hepatitis A
The inactivated virus vaccines currently licensed in the United States are the single-antigen HAVRIX® and VAQTA® and the combination of HAV and hepatitis B virus (HBV) antigen vaccine TWINRIX®. Both single-antigen vaccines are available for pediatric and adult use while the TWINRIX is indicated for adults only (Table 26-3). The differences in the vaccines are in the use of a preservative and in expression of antigen content. VAQTA is formulated without a preservative and uses units of HAV antigen to express potency. HAVRIX and TWINRIX use 2-phenoxyphenol as a preservative and antigen content is expressed as enzyme-linked immunosorbent assay (ELISA) units.7 Although high seroconversion rates of ≥94% are achieved with the first dose, VAQTA and HAVRIX recommend a booster shot to achieve the highest possible antibody titers. Although seroconversion exceeds 90% for HAV after the first dose of TWINRIX, the full three-dose series is required for maximal HBV seroconversion. An accelerated dosing schedule is available but requires a total of four doses for optimal response. The combined vaccine offers the advantage of immunization against both types of hepatitis in a single vaccine.
TABLE 26-3 Recommended Dosing of Hepatitis A Vaccines
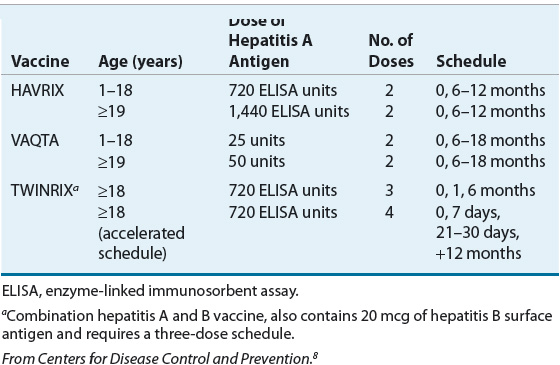
In situations of postexposure prophylaxis, either the vaccine or Ig can be used. The use of the vaccine is advantageous as vaccination confers the benefit of long-term immunity against HAV; however, experience in patients aged >40 years or with underlying medical conditions is limited.8 Both vaccines may be given concomitantly with Ig and the two brands are interchangeable for booster shots.7
Vaccine is recommended for international travel to areas of high or intermediate endemicity and can be given regardless of scheduled dates of departure. For older patients, immunocompromised, or any patients with chronic liver disease or any other chronic medical conditions traveling within 2 weeks, both Ig and vaccine are recommended.8
The most common side effects of the vaccines include soreness and warmth at the injection site, headache, malaise, and pain. More than 65 million doses of the vaccine have been administered and despite routine monitoring for adverse events, there are no data to suggest a greater incidence of serious adverse events among vaccinated people compared with nonvaccinated. The vaccine is considered safe.7
Immunoglobulin
Ig is used when preexposure or postexposure prophylaxis against HAV infection is needed in persons for whom vaccination is not an option. Vaccination is preferred for multiple reasons, including that it induces active immunity and therefore a longer time of protection against HAV than Ig. Ig is preferred for children <12 months of age and for postexposure prophylaxis in patients aged >60 years, patients with chronic liver disease, and persons allergic to any part of the vaccine. Among this patient population, Ig is preferred because it is effective and these populations are the most likely to experience fulminant hepatitis and mortality secondary to active HAV infections.
A sterile preparation of concentrated antibodies against HAV, Ig provides protection by passive transfer of antibody. Ig is most effective if given in the incubation period of the infection. Receipt of Ig within the first 2 weeks of infection will reduce infectivity and moderate the infection in 85% of patients. Patients who receive at least one dose of the HAV vaccine at least 1 month earlier do not need preexposure or postexposure prophylaxis with Ig.7 Ig is available as both an IV and IM injection, but for HAV exposure, only the IM is used. If given to infants or pregnant women, the thimerosal-free formulation should be used.
Serious adverse events are rare. Anaphylaxis has been reported in patients with IgA deficiency. Patients who had an anaphylaxis reaction to Ig should not receive it. There is no contraindication for use in pregnancy or lactation.
Dosing of Ig is the same for adults and children. For postexposure prophylaxis and for short-term preexposure coverage of <3 months, a single dose of 0.02 mL/kg is given intramuscularly. For long-term preexposure prophylaxis of ≤5 months, a single dose of 0.06 mL/kg is used. Either the deltoid or gluteal muscle may be used. In children younger than 24 months of age, Ig can be given in the anterolateral thigh muscle.7
For people who were recently exposed to HAV and who had not been previously vaccinated, postexposure prophylaxis with vaccination is preferred for most patients. Ig prophylaxis is preferred in the following situations: patients are <12 months of age or >40 years of age, are immunocompromised, have chronic liver disease or have underlying medical conditions, or for whom vaccine is contraindicated.8
Ig can be given concomitantly with the HAV vaccine. Although the antibody titer will be lower than if the vaccine were administered alone, the response is still protective and coadministration should be considered for the advantages of long-term HAV protection. However, Ig can interfere with the response of other live, attenuated vaccines and should be delayed.
Personalized Pharmacotherapy
Vaccine efficacy may be reduced in certain patient populations. In HIV-infected patients, greater immunogenic response may correlate with higher baseline CD4 cell counts. Response to the HAV vaccine as determined by detection of anti-HAV after vaccination found that among HIV patients, patients with CD4 counts <200 cells/mm3 (<200 × 106/L) at vaccination had a reduced response rate.9
HEPATITIS B
Hepatitis B is highly infectious, approximately 50 to 100 times more so than HIV.10 Although a vaccine was made available in 1981, HBV has acutely infected more than 2 billion people globally, leading to chronic infection in more than 240 million people.10 Chronic infection with HBV is a major public health issue as it serves as a reservoir for continued HBV transmission and poses a significant risk of death resulting from liver disease. More than 600,000 people per year die as a result of liver cirrhosis and hepatocellular carcinoma (HCC). In the United States, estimates of prevalence and incidence of viral hepatitis are difficult because there is no national chronic hepatitis surveillance program.1
Epidemiology
According to the World Health Organization (WHO), an estimated 2 billion people have been infected with HBV; only 12% of the global population lives in an area of low prevalence for hepatitis B, defined as an area where <2% of the population is hepatitis B surface antigen (HBsAg) positive.10 Prevalence can vary regionally; however, areas commonly associated with high infectivity rates include sub-Saharan Africa, most of Asia, as well as the Amazon and southern parts of Eastern and Central Europe.10 Areas of high prevalence, approximately 45% of the global population, are of special concern because most infections are of infants and children and >90% of cases lead to a chronic carrier state. Myths and misinformation about hepatitis B abound and can result in discrimination and social injustice.1 There are approximately 1.4 million chronically infected HBV people in the United States. Rates of acute infection in the United States continue to decline and in 2010, an estimated 38,000 people developed new infections. In 2010, the highest incidence rate was among persons aged 30 to 39 years and among non-Hispanic blacks. Data from a limited chronic surveillance program indicate Asian/Pacific Islanders account for the highest proportion of chronic HBV infections.2 Annually, 3,000 people die from chronic liver disease attributable to HBV.1
HBV is transmitted sexually, parenterally, and perinatally. In areas of high HBV prevalence, perinatal transmission from mother to infant at birth is most common, whereas in areas of intermediate prevalence, horizontal transmission from child to child is most common. Sexual contact, both homosexual and heterosexual, and injection-drug use are the predominant forms of transmission in low endemic countries such as the United States.10 Concentration of HBV is high in blood, serum, and wound exudates of infected persons. Transmission occurs via blood-to-blood contact or semen or vaginal fluid of an infected person. The virus can be stable in the environment for at least 7 days and can cause infection during this time but is not spread via contaminated food or water and is not casually transmitted in the workplace.10 In the United States in 2010, no risk factor could be identified for the majority of acute infections with HBV. Among patients with identifiable risk factors, the most common risk continues to be sexual contact, specifically multiple sexual partners, MSM, and sexual contact with a known HBV-positive person. Sexual contact was a consistent risk among all patients but especially among those aged 45 years or younger. Other known risk factors include IDU and household contact of HBV-positive person.11
PERSONS AT HIGH RISK FOR HBV: RECOMMENDED SCREENING
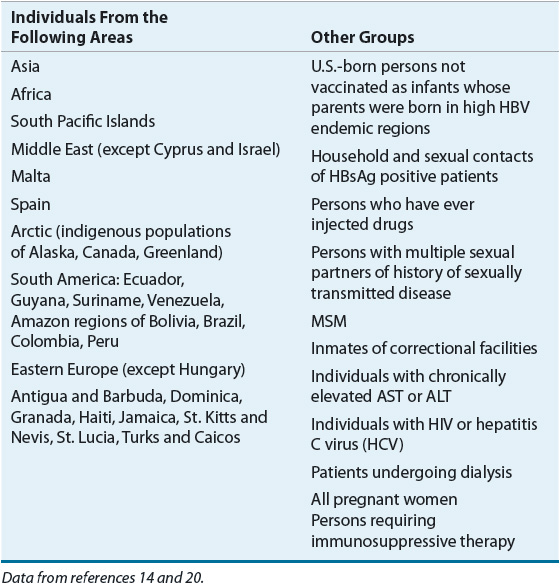
The mode of transmission has clinical implications because chronic infections are associated with infection acquired in younger patients, especially those infected perinatally and in early childhood.10
Clinical Controversy…
Etiology
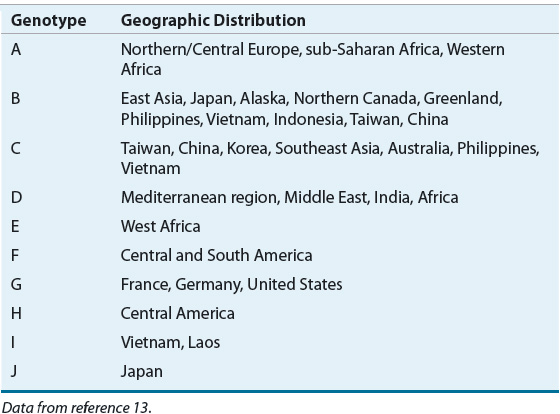
The HBV is a DNA virus that preferentially replicates within the liver.12 There are at least 10 HBV genotypes (A to J) with distinct geographic and ethnic distribution (Table 26-4). Genotype prevalence may depend on mode of transmission as types B and C are found in areas where vertical transmission is the primary mode of infection.13 Additionally, various subtypes of genotypes exist with varying clinical outcomes. Correlations between clinical outcomes and HBV genotypes suggest infections with genotype C are associated with more severe liver injury, including liver cirrhosis and progression to HCC. Noted limitations of studies are frequently small sample sizes and a predominance of research from Asia, primarily comparing genotypes B and C.13 Nonetheless, risk of more severe liver fibrosis was significantly higher in HBV genotype A-, C-, and D-infected patients. Genotype B may be more benign because it is associated with faster seroconversion, although clinical studies suggest genotype A may have equivalent, if not higher, rates of seroconversion.13 Patients with genotype C tend to have persistently higher viral DNA levels. Higher viral DNA levels are associated with increased incidence of cirrhosis and HCC. Resistance mutations may contribute to genotype virulence and hence impact severity of liver disease in infection.13–15 Testing for HBV genotype is not currently recommended for clinical practice.14
TABLE 26-4 Worldwide Distribution of Hepatitis B Virus Genotypes
Pathophysiology
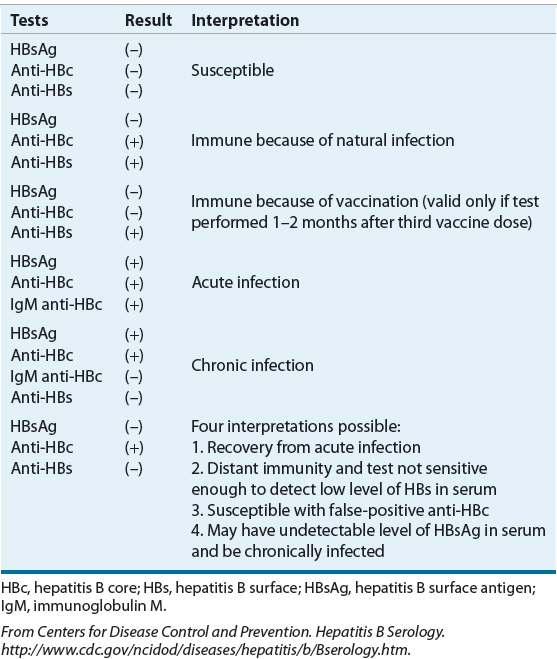
On infection, replication of the virus begins by attachment of the virion to the hepatocyte cell surface receptors. The particles are transported to the nucleus where the DNA is converted into closed, circular DNA that serves as a template for pregenomic RNA. Viral RNA is then transcribed and transported back to the cytoplasm where it can alternatively serve as a reservoir for future viral templates or bud into the intracellular membrane with the viral envelope proteins and infect other cells.13 The viral genome has four reading frames coding for various proteins and enzymes required for viral replication and spread. Several of these proteins are used diagnostically (Table 26-5). The HBsAg is the most abundant of the three surface antigens and is detectable at the onset of clinical symptoms. Its persistence past 6 months after initial detection corresponds to chronic infection and indicates an increased risk for cirrhosis, hepatic decompensation, and HCC. Development of antibody to HBsAg (anti-HBsAg) confers immunity to the virus and clearance of HBsAg is associated with favorable outcomes.16 The precore polypeptide encodes for the secretory protein hepatitis B e antigen (HBeAg) and the hepatitis B core antigen (HBcAg) proteins. HBeAg is present in an acute infection and is replaced by antibodies (anti-HBeAg) once an infection is resolved. HBeAg was assumed to be a marker of viral replication and infectivity; however, it is now known that some viral mutants exist that are unable to have or have downregulated expression of HBeAg, although their ability to replicate is not affected.14 HBeAg-negative mutants pose a particular clinical challenge because they are refractory to treatment. The HBcAg is a nucleocapsid protein that, when expressed on hepatocytes, promotes immune-mediated cell death. High levels of antibodies (IgM anti-HBcAg) are detectable during acute infections. Patients who respond to vaccine will have anti-HBsAg only.7
TABLE 26-5 Interpretation of Serologic Tests in Hepatitis B Virus
HBV itself does not seem to be pathogenic to cells; rather, it is thought that the immune response to the virus is cytotoxic to hepatocytes.13 The immune response is critical to viral clearance. If the response is weak, chronic infection is likely. Liver injury is likely caused by secondary, nonspecific inflammation activated by the initial cytotoxic lymphocyte response and as an attempt by the immune system to clear the virus by destroying HBV antigen-presenting hepatocytes. Destruction of hepatocytes results in release of circulating, and hence increased, ALT levels.
Cirrhosis
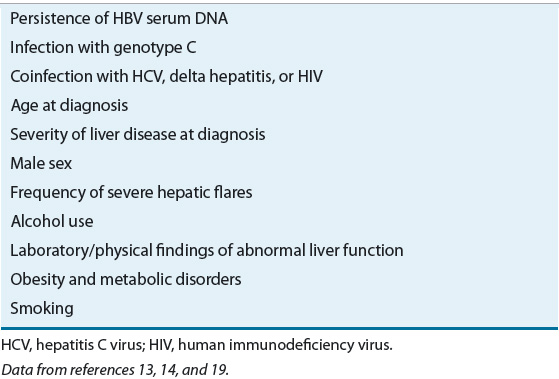
Cirrhosis results as the liver attempts to regenerate while in an environment of persistent inflammation. Most patients with compensated cirrhosis either are asymptomatic or have mild symptoms of epigastric pain. During cirrhosis, the liver enters a cycle of ongoing liver damage, fibrosis, and attempts at regeneration. The classical appearance of a small and knobby liver reflects the irreversible effect of nodules of regenerating cells integrated with infiltrates of inflammation-induced fibrous tissue. Both viral and clinical factors affect the outcome of cirrhosis (Table 26-6). Cirrhosis develops at an annual incidence rate of 2.1% to 3.5%.13 The development of cirrhosis is mostly insidious and patients can remain stable for years before disease progression. An estimated 20% of all chronic hepatitis B patients develop complications of hepatic insufficiency and portal hypertension as their compensated cirrhosis progresses to decompensated cirrhosis within a 5-year period.14
TABLE 26-6 Factors Associated with Hepatitis B Virus (HBV) Cirrhosis and Disease Progression
Hepatocellular Carcinoma
HBV is a known risk factor for the development of HCC and in areas of high HBV endemicity, a major complication of the infection.10 The development of HCC can be insidious, occurring in the absence of cirrhosis or in the presence of clinically silent, compensated cirrhosis. Many patients with HCC have no signs of cirrhosis.14 The virus itself is not likely the causative agent of the cancer. In most cases, HCC develops after years of inflammatory processes provoked by ongoing HBV infection. Compared with HCV, however, HBV does seem to provoke a more direct carcinogenic effect as evidenced by its presence in less severe liver disease, and among patients with advanced HCC, HBV infection is associated with a worse survival rate.17 Several factors influence the development of HCC, as well as predict survival (see Table 26-6). HCC is more prevalent in males; in older patients; in patients coinfected with HCV or delta hepatitis; and in patients with serologic markings of past or present HBV infection, preexisting cirrhosis, or continued alcohol ingestion. Risks for death and decompensation increase with underlying liver disease. Other host-specific or environmental factors may impact the course of liver disease. Persistently elevated HBV DNA levels (≥10,000 copies/mL [≥10 × 106 copies/L]) predict HCC development, even after adjusting for sex, age, cigarette smoking, alcohol consumption, HBeAg status, ALT level, and liver cirrhosis.18 Smoking is a risk factor among European and Asian patients.19,20 HBeAg status is not a risk factor. In otherwise healthy patients without coinfection or who do not have HCC or decompensation at the time of seroclearance, HBsAg seroclearance does predict a favorable long-term outcome.16
Clinical Presentation
![]() The clinical symptoms and course of an HBV infection are indistinguishable from other types of viral hepatitis. Several phases of an HBV infection exist and are dynamic.15 During the initial or acute phase of an HBV infection in adults and older children, the HBV enters a 4- to 10-week incubation period, during which antibodies toward the HBV core are produced and the virus replicates profusely. Active viral replication results in high serum HBV DNA levels and HBeAg secretion. ALT levels may rise slightly, but most patients will remain asymptomatic. Symptoms, if they do occur, include fever, anorexia, nausea, vomiting, jaundice, dark urine, clay-colored or pale stools, and abdominal pain. Most neonates and children are anicteric and have no clinical symptoms; many adults are also asymptomatic.10 HBsAg does not become detectable until after significant viremia. The initial phase is considered immunotolerant because no hepatic injury is sustained, as evidenced by generally normal ALT levels, and the virus replicates profusely. Patients are highly infectious during this time.15 In perinatally acquired infections, and in young children, the phase can last for decades—until adulthood.15 Infected children pose a particular risk because they are often asymptomatic, undiagnosed, and highly infectious.
The clinical symptoms and course of an HBV infection are indistinguishable from other types of viral hepatitis. Several phases of an HBV infection exist and are dynamic.15 During the initial or acute phase of an HBV infection in adults and older children, the HBV enters a 4- to 10-week incubation period, during which antibodies toward the HBV core are produced and the virus replicates profusely. Active viral replication results in high serum HBV DNA levels and HBeAg secretion. ALT levels may rise slightly, but most patients will remain asymptomatic. Symptoms, if they do occur, include fever, anorexia, nausea, vomiting, jaundice, dark urine, clay-colored or pale stools, and abdominal pain. Most neonates and children are anicteric and have no clinical symptoms; many adults are also asymptomatic.10 HBsAg does not become detectable until after significant viremia. The initial phase is considered immunotolerant because no hepatic injury is sustained, as evidenced by generally normal ALT levels, and the virus replicates profusely. Patients are highly infectious during this time.15 In perinatally acquired infections, and in young children, the phase can last for decades—until adulthood.15 Infected children pose a particular risk because they are often asymptomatic, undiagnosed, and highly infectious.
The immunoactive phase marks a decrease in HBV DNA levels with ongoing secretion of HBeAg. Patients are symptomatic with intermittent flares of hepatitis and marked increases in ALT levels. More frequent flares are associated with disease progression and reflect host immune response against HBV-infected hepatocytes, increased cell death in an attempt to clear the virus.13,19 The phase can last a few weeks in acute disease, and for years in patients with chronic disease. As the host immune system attempts to gain control of the infection by stopping active viral replication, serum HBV DNA levels drop to undetectable, ALT levels normalize, and liver necroinflammation resolves.13
If the infection is self-limiting, HBV DNA quickly subsides, HBeAg disappears within weeks, and HBsAg usually resolves within 4 months. The final phase is seroconversion and is defined by the replacement of HBeAg with anti-HBeAg. Factors favoring seroconversion include female sex, older age, biochemical activity, and genotype. Flares of hepatitis with ALT levels >5 times the upper limits of normal, compared with <5 times the upper limits of normal, correspond to increased immune system activity and precede seroconversion.
Chronic HBV
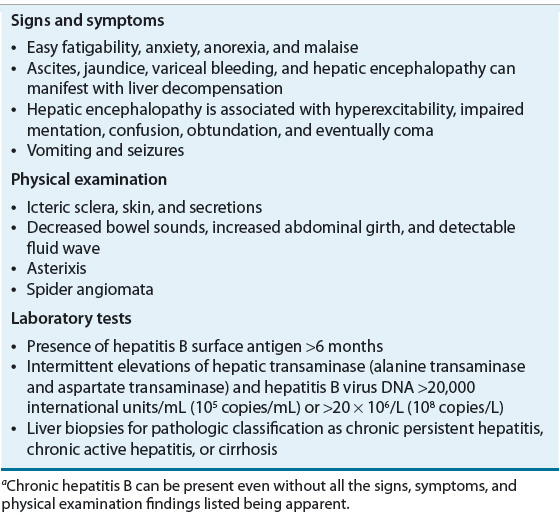
![]() Patients who continue to have detectable HBsAg for more than 6 months have chronic HBV.13 Table 26-7 lists the clinical features of chronic hepatitis B. The most predictive factor for developing a chronic infection is age. Perinatal infections almost always result in chronic infections because of immune tolerance to the virus. Risks of chronicity decline to a rate of 30% in infants and to less than 5% of acute adult infections.14
Patients who continue to have detectable HBsAg for more than 6 months have chronic HBV.13 Table 26-7 lists the clinical features of chronic hepatitis B. The most predictive factor for developing a chronic infection is age. Perinatal infections almost always result in chronic infections because of immune tolerance to the virus. Risks of chronicity decline to a rate of 30% in infants and to less than 5% of acute adult infections.14
TABLE 26-7 Clinical Presentation of Chronic Hepatitis Ba
Chronic infections can be controlled in many cases, but cure is not possible because the HBV template is integrated into the host genome. In patients with recurring cycles of viral expression and host immune response, progressive liver damage ensues.21 Patients can be divided into two types of chronic hepatitis B: those who are HBeAg positive and those who are HBeAg negative. The ability to express HBeAg by the virus differentiates the two types of chronic infection. Patients are considered to be in the “immune-tolerant” phase when HBeAg is positive, high serum HBV DNA levels are detected, and ALT levels are normal. Typically these patients were infected early in life and develop elevated ALT levels later in life. Spontaneous HBeAg clearance is possible and is associated with older age, higher ALT, and infection with HBV genotype B.14
Patients who are HBeAg negative can be further subdivided into the active or inactive carrier. HBeAg-negative chronic HBV patients who are active carriers have a worse clinical course with a very low rate of spontaneous remission. Patients may have long periods of disease remission, but recurring flares of hepatitis with increased frequency and severity can progress to cirrhosis and HCC. In contrast, HBeAg-negative chronic HBV patients who are inactive carriers have detectable HBsAg and anti-HBeAg, normal ALT, and either low or undetectable levels of HBV DNA. This patient population usually experiences a more benign course of disease, with the possibility of long-term remission, even seroconversion, although reactivation is possible with the progression to cirrhosis and HCC. Up to 20% of patients in the inactive carrier state may revert to detectable HBeAg, emphasizing the need for lifelong followup to confirm quiescence.14
Reactivation of hepatitis B, defined as the recurrence or abrupt rise in HBV replication by an increase in serum HBV DNA of at least 1 log10 and a marked increase in transaminase levels, can occur and is well described in the literature in patients receiving cancer chemotherapy, steroids, and other immunosuppressive agents.22,23 Reactivation can occur in anyone with a prior or current HBV exposure, but patients who are HBsAg positive are most likely to experience a reactivation.23 The causes of reactivation include spontaneous mutations of the virus that allow it to escape immune control, development of resistance to HBV drug therapy or the cessation of HBV therapy, or changes in immunity, such as those that occur in patients undergoing immunosuppressive therapies or coinfection with HIV.
The CDC recommends testing for hepatitis B for all patients who are to receive chemotherapy or other immunosuppressive agents.
HBV Mutations
![]() Among the DNA viruses, HBV is notable for its significantly higher mutation rate.24 Long-term therapy is problematic because of the high likelihood of developing viral resistance. One of the most common mutations consists of a nucleotide substitution either preventing or causing downregulation of the production of HBeAg. The mutation results in a chronic infection that may have a poorer long-term prognosis. Typically, the mutation emerges during the infection and represents a later stage in the course of chronic HBV infection with more advanced liver disease.
Among the DNA viruses, HBV is notable for its significantly higher mutation rate.24 Long-term therapy is problematic because of the high likelihood of developing viral resistance. One of the most common mutations consists of a nucleotide substitution either preventing or causing downregulation of the production of HBeAg. The mutation results in a chronic infection that may have a poorer long-term prognosis. Typically, the mutation emerges during the infection and represents a later stage in the course of chronic HBV infection with more advanced liver disease.
The selective pressures of the L-nucleoside analog antivirals, including lamivudine, cause the YMDD mutation. The mutation is associated with the active site of the DNA polymerase and causes an altered active site. The incidence of lamivudine resistance increases with each subsequent year of therapy and may be associated with a more severe disease progression.14 An added risk of developing resistance is the retention of the mutation within the virus even 4 years after lamivudine therapy.25 Cross-resistance of lamivudine-resistant mutants to telbivudine has been demonstrated and patients treated with lamivudine and telbivudine showed resistance mutations to both drugs. Telbivudine-specific resistance can also occur at rates lower than that seen in lamivudine.26 Other mutations include resistance to adefovir and entecavir. Resistance to adefovir is associated with substitutions of aspargine by threonine (rtN236T) and substitution of alanine by valine or threonine (rtA181V/T), as well as other possible mutations to the HBV polymerase gene. The addition of lamivudine in adefovir resistance may overcome resistance, although the optimal management of either adefovir- or entecavir-resistant strains is not clear.13–15,24
Prevention of Hepatitis B
The development of the HBV vaccine represented the first vaccine against a major human cancer.10 Despite the availability of the HBV vaccine in 1982, rates of HBV did not decline in the early 1980s. Initial declines in incidence were likely attributable to behavioral changes among high-risk groups as a result of the acquired immune deficiency syndrome (AIDS) epidemic. A 94% decline in rates between 1990 and 2004 was seen in children and adolescents, which began with the initiation of screening of pregnant women and subsequent immunizations of infants and recommendations set forth in the 1990s to immunize adolescents. Regulations enacted by Occupational Safety and Health Administration (OSHA) further reduced overall U.S. rates by 75%.12
![]() Prophylaxis against HBV can be achieved by vaccination or by passive immunity in postexposure cases with hepatitis B Ig. Vaccination is the most effective strategy to prevent infection and a comprehensive vaccination strategy has been implemented in the United States (Table 26-8). Vaccines use HBsAg for the antigen via recombinant DNA technology using yeast to prompt active immunity. More than 60 million adolescents and more than 40 million infants and children have received an HBV vaccine in the United States since 1982. The vaccine is considered safe. Since 2000, vaccines licensed in the United States contain either none or trace amounts of thimerosal as a preservative. Available vaccines include two single-antigen products and three combination products. The two single-antigen products are Recombivax HB and Engerix-B. TWINRIX is a combination vaccine for HAV and HBV in adults. Comvax and Pediarix are used for children and are used for HBV along with other scheduled vaccines.
Prophylaxis against HBV can be achieved by vaccination or by passive immunity in postexposure cases with hepatitis B Ig. Vaccination is the most effective strategy to prevent infection and a comprehensive vaccination strategy has been implemented in the United States (Table 26-8). Vaccines use HBsAg for the antigen via recombinant DNA technology using yeast to prompt active immunity. More than 60 million adolescents and more than 40 million infants and children have received an HBV vaccine in the United States since 1982. The vaccine is considered safe. Since 2000, vaccines licensed in the United States contain either none or trace amounts of thimerosal as a preservative. Available vaccines include two single-antigen products and three combination products. The two single-antigen products are Recombivax HB and Engerix-B. TWINRIX is a combination vaccine for HAV and HBV in adults. Comvax and Pediarix are used for children and are used for HBV along with other scheduled vaccines.
TABLE 26-8 Recommendations for Hepatitis B Virus (HBV) Vaccination




