Vertical Banded Gastroplasty Revision
Eric J. DeMaria
James W. Maher
Introduction
Although vertical banded gastroplasty (VBG) remains one of the two procedures recognized by the National Institutes of Health as safe and effective in promoting and maintaining long-term weight loss in the severely obese, it has fallen into disfavor and today is a rarely performed procedure in the United States. One of the values of VBG is its ability to induce weight loss without affecting the normal sequence of digestive events or inducing malabsorption. VBG however, like adjustable gastric banding, which is similar in many regards, seems to be more dependent on the patient’s ability to maintain lifelong alterations in their eating habits. These changes include avoiding high-calorie liquids and such calorie-rich foods as cake, cookies, and other junk foods that undergo substantial liquefaction in the mouth and thus arrive in the VBG pouch as a liquid slurry that is not substantially restricted by the outlet. This dependence on patient behavior is the likely source of the higher failure rate seen after VBG than after procedures incorporating a degree of malabsorption such as Roux Y gastric bypass or biliopancreatic bypass and is probably the most common reason for VBG failure. Revision in patients who exhibit these maladaptive eating behaviors is unlikely to be effective in promoting long-term weight loss unless significant malabsorption is induced or lifelong behavioral modifications (described above) can be ensured. Further, revision entails higher risks than primary surgery and is ill advised unless there is a high likelihood of success. These circumstances make it necessary that the patient not change their dietary habits. One technique is to make the patient maintain a food diary for several weeks, measuring portions to enable an accurate analysis of dietary habits by a bariatric dietician. Another is to require that candidates for VBG conversion to an alternative procedure demonstrate compliance with a 1500 to 2000 calorie diet avoiding all caloric liquids, as well as cookies, cake, candy, and other high-calorie snacks. One 12-ounce can of Coca-Cola or other sweetened soda or juice per day yields the equivalent of 17 pounds of fat calories in one year. There are no restrictive operations that can overcome this type of challenge.
Modes of Failure and Strategy
There are also a number of ways in which VBG can fail that are not dependent on patient behavior.
Staple line dehiscence was a major problem early in the development of VBG. The initial iteration of the operation involved placing two to three applications of the TA-90 stapler from the window to the angle of His. This technique did not reliably produce healing of the vertical staple line and dehiscences led to a revision rate of 2% per year early on. This problem was largely solved by introduction of the TA-90B stapler, which placed four closely spaced staggered rows of staples that did heal solidly. The revision rate following this innovation dropped precipitously but sporadic dehiscences are still occasionally seen, particularly in postpartum patients (the reason for this association is unknown). Small dehiscences do not substantially impede the restrictive effects of the operation but dehiscences larger than 1 cm may lead to both weight gain and gastroesophageal reflux disease. It is possible to restaple a dehisced staple line; however, reapplying staples to a thickened, scarred stomach wall may be associated with not only another dehiscence but leaks from the staple lines. The success rate in resuming and maintaining weight loss with reapplication of staples is also generally less satisfactory than substitution of a procedure that induces malabsorption such as gastric bypass or biliopancreatic diversion. Nevertheless, patients who had a good result prior to the staple dehiscence typically respond well to reconstruction of the VBG.
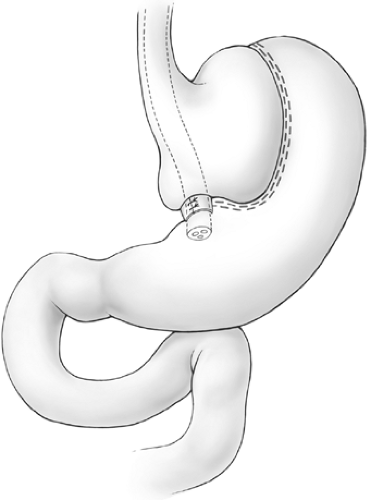
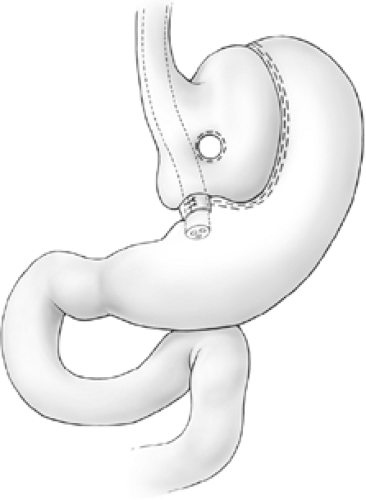
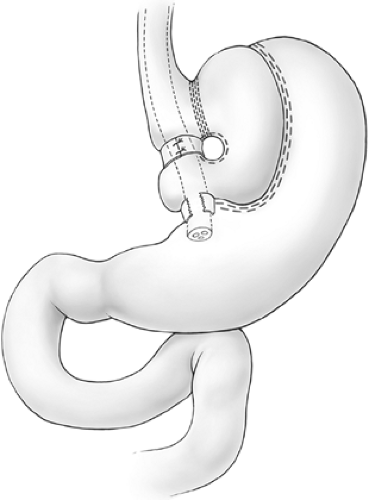
Pouch enlargement may be secondary to repetitive gorging by the patient to the point of emesis or to stricture of the banded segment, which is often present for years before investigation as patients are frequently blamed for dietary indiscretion when they report frequent vomiting after meals. Inclusion of an excessive amount of fundus by the surgeon at the initial surgery is another known cause of pouch enlargement. The surgeon should remain cognizant that part of the function of the fundus is to dilate to accommodate ingestion of larger meals. Thus, inclusion of a significant amount of fundus may promote pouch dilation. The initial vertical staple line should be placed precisely at the angle of His. Pouch enlargement, with or without stricture, may be associated with weight gain, reflux, nausea, and vomiting. Dietary indiscretion plays a role in weight gain as most patients gravitate toward a liquid calorie-dense diet or other high-calorie junk foods such as cake that undergo liquefaction in the mouth and arrive in the pouch in the form of a liquid slurry that is more likely to pass through the outlet. As the pouch enlarges, the banded stoma is no longer dependent, and, since this portion of the stomach has no active peristalsis, gastric stasis is promoted (Fig. 1). Correction of the reflux and stasis may be accomplished by simply removing a portion of the Marlex band and dilating the pouch outlet. This approach, however, is almost invariably accompanied by regain of lost weight. Band removal or division may be appropriate in severely debilitated patients where gastric stasis has led to aspiration pneumonia, but, in general, patients should have some sort of procedure to maintain or resume their weight loss. Mason has described the so-called Faberge technique (referring to the “egg within an egg” works of art of the Romanov dynasty) of constructing a second appropriately sized VBG within the initial enlarged VBG. Such procedure can be constructed either by using another circular window and vertical stapled partition within the small pouch (Figs. 2 and 3) or, more simply, by banding the outlet with encircling nonabsorbable sutures (Fig. 4). This technique may be quite successful with selected patients; however, most individuals with an enlarged pouch have this problem because of an inability to permanently change their eating habits following the first operation. Further, this procedure involves restapling the previously stapled thickened fundus, which may often be quite adherent posteriorly to the pancreas and retrogastric structures. In this situation, the authors prefer to substitute a procedure that induces some degree of malabsorption: either a gastric bypass or a biliopancreatic diversion (Scopinaro’s procedure).
Erosion of the Marlex band into the gastric lumen is sometimes seen in patients following VBG and may be associated with either reflux, pain, and vomiting or microcytic anemia. The etiology is unknown but may be more common in patients ingesting
anti-inflammatory medications. Some patients may be completely asymptomatic and no therapy is necessary if this is the case. The eroded band is usually partially intraluminal and partially extraluminal. Sometimes this can be treated by endoscopic excision of the intraluminal portion of the mesh, avoiding the need for reoperation. This technique requires that heavy biopsy forceps be threaded the loop of a colonoscopy snare. The endoscopist grasps the mesh with the forceps and, with traction on the mesh, the snare is snugged down around the base of the mesh. The cautery is then activated and the mesh transected. Many times excision of the protruding mesh renders the patient asymptomatic. If this technique is not successful, the patient should have transgastric excision of the mesh through a gastrotomy. This can be combined with other bariatric procedures, if indicated.
anti-inflammatory medications. Some patients may be completely asymptomatic and no therapy is necessary if this is the case. The eroded band is usually partially intraluminal and partially extraluminal. Sometimes this can be treated by endoscopic excision of the intraluminal portion of the mesh, avoiding the need for reoperation. This technique requires that heavy biopsy forceps be threaded the loop of a colonoscopy snare. The endoscopist grasps the mesh with the forceps and, with traction on the mesh, the snare is snugged down around the base of the mesh. The cautery is then activated and the mesh transected. Many times excision of the protruding mesh renders the patient asymptomatic. If this technique is not successful, the patient should have transgastric excision of the mesh through a gastrotomy. This can be combined with other bariatric procedures, if indicated.
Description of Procedures
Simple Repair of Staple Line Dehiscence
This approach, while simple in concept, may be challenging in its application. As soon as the adhesions to the abdominal wall are separated, a table-mounted retractor should be mounted for stable retraction of the
abdominal wall and the patient placed in steep reversed Trendelenburg position (a footboard should always be placed to avoid slippage of the patient). The next step is to separate the liver from the stomach. This may be relatively easy if the previous surgeon was considerate enough to cover the Marlex with omentum in the recommended fashion. However, all too often this may be somewhat difficult. There is no substitute for careful sharp dissection here. We routinely place the endoscope through the banded outlet of the pouch prior to initiating dissection. This allows the surgeon to palpate not only the vertical pouch but also the area of the band. This is a great aid in the dissection of the anterior wall of the stomach. After freeing the anterior wall the liver should be retracted superiorly and, if possible, the pouch window opened (this has usually contracted to quite a small opening). The lesser curve access to the lesser sac inferior to the left gastric artery through the gastrohepatic omentum is typically obliterated. It may be advisable to open the gastrocolic omentum outside the gastroepiploic vessels and dissect the posterior wall of the stomach from this aspect. Again, careful dissection is important to avoid injury to the pancreas or posterior gastric wall. At this point, with both anterior and posterior gastric walls free, one can try and develop a connection between the two areas at the angle of His.
abdominal wall and the patient placed in steep reversed Trendelenburg position (a footboard should always be placed to avoid slippage of the patient). The next step is to separate the liver from the stomach. This may be relatively easy if the previous surgeon was considerate enough to cover the Marlex with omentum in the recommended fashion. However, all too often this may be somewhat difficult. There is no substitute for careful sharp dissection here. We routinely place the endoscope through the banded outlet of the pouch prior to initiating dissection. This allows the surgeon to palpate not only the vertical pouch but also the area of the band. This is a great aid in the dissection of the anterior wall of the stomach. After freeing the anterior wall the liver should be retracted superiorly and, if possible, the pouch window opened (this has usually contracted to quite a small opening). The lesser curve access to the lesser sac inferior to the left gastric artery through the gastrohepatic omentum is typically obliterated. It may be advisable to open the gastrocolic omentum outside the gastroepiploic vessels and dissect the posterior wall of the stomach from this aspect. Again, careful dissection is important to avoid injury to the pancreas or posterior gastric wall. At this point, with both anterior and posterior gastric walls free, one can try and develop a connection between the two areas at the angle of His.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree