aNot an FDA-approved indication.
IU, anti-Xa units; for enoxaparin, 1 mg = 100 anti-Xa units.
Caution with the use of fondaparinux, tinzaparin, dalteparin, or enoxaparin for pregnancy, morbid obesity, or end-stage renal disease (CrCl <30 mL/minute); anti-Xa level monitoring is recommended in these settings.
Unfractionated Heparin
- UFH comes from porcine intestinal mucosa, and it mainly works indirectly by catalyzing the inactivation of thrombin and factor Xa by antithrombin.
- At usual doses, UFH prolongs the thrombin time and aPTT and has a small effect on the PT/INR.
- Because the anticoagulant effects of UFH normalize within hours of discontinuation and protamine sulfate reverses it even faster, UFH is the anticoagulant of choice for patients with increased risk of bleeding.
- Abnormal renal function does not typically affect UFH dosing.
- For therapeutic anticoagulation, UFH is usually administered IV with a bolus followed by continuous infusion, but this is almost exclusively done in the inpatient setting.
- Treatment doses of UFH may be administered SC, initial dose of 333 U/kg SC, followed by a fixed unmonitored dose of 250 U/kg every 12 hours.20 However, this regimen requires large-dose injections and is used infrequently.
- Monitored and adjusted SC UFH may be used to treat VTE, but this is not commonly done since many easier and potentially better alternatives exist. In addition, the therapeutic range (approximately 60 to 90 seconds) for aPTT is not standardized.
Low Molecular Weight Heparin
- LMWHs are produced by chemical or enzymatic cleavage of UFH, and they have a similar mechanism of action as UFH.
- Since LMWHs inactivate factor Xa to a greater extent than they do to thrombin (factor IIa), LWMHs minimally prolong the aPTT.
- Extensive clinical trials have confirmed the efficacy and safety of weight-based SC LMWH for the treatment of VTE.
- Factor Xa monitoring is not recommended, except in special circumstances where dose adjustments may be necessary, such as renal dysfunction, morbid obesity, cachexia, and pregnancy. Peak factor Xa levels, measured 4 hours after an SC dose, should be 0.6 to 1 IU/mL for q12-hour dosing and 1 to 2 IU/mL for q24-hour dosing.21
- Different LMWH preparations have different dosing recommendations (Table 14-1).
- Given the renal clearance of LMWHs, they are generally avoided in patients undergoing dialysis.
- Patients with a CrCl of 15 to 30 mL/minute require dose adjustments (e.g., enoxaparin 1 mg/kg once daily instead of twice daily).
- Patients with cancer may have reduced recurrent VTE when treated long-term with LMWH rather than warfarin (or other coumarins). For example, subcutaneous dalteparin at dose of 200 IU/kg daily for 1 month, followed by a daily dose of 150 IU/kg, has been used successfully in patients with VTE and cancer.22
- Protamine only partially reverses LMWH.
Fondaparinux
- Fondaparinux, a synthetic pentasaccharide that is structurally similar to a region of the heparin molecule, binds antithrombin and functions as a selective indirect inhibitor of factor Xa.
- Because fondaparinux inhibits factor Xa and does not inhibit thrombin, it does not significantly prolong the aPTT.
- Large clinical trials have confirmed the efficacy and safety of weight-based subcutaneously dosed fondaparinux for the treatment of VTE.23,24
- Similar to the LMWHs, fondaparinux does not require factor Xa monitoring, except for patients with significant renal dysfunction.
- The recommended dose for VTE therapy ranges from 5 to 10 mg SC daily, depending on weight (Table 14-1).23,24
Oral Direct Xa Inhibitors
- As compared to warfarin, the oral direct (i.e., do not require antithrombin) factor Xa inhibitors rivaroxaban and apixaban have a faster onset, shorter half-life, wider therapeutic window, and more predictable pharmacokinetics.
- In general, the features of the oral direct factor Xa inhibitors allow for fixed dosing without INR monitoring, though they may increase the INR and aPTT in a nonlinear fashion.
- Since both rivaroxaban and apixaban are substrates of cytochrome P450 (CYP) 3A4, strong inhibitors (e.g., clarithromycin) and inducers (e.g., St. John’s wort) alter plasma concentrations.
Rivaroxaban
- The dose of oral rivaroxaban for acute VTE is 15 mg twice daily × 3 weeks, then 20 mg daily. Therapy can be started at the time of diagnosis, without concomitant use of UFH or LMWH. Therapy can also begin after transition from initial treatment with a parenteral agent (e.g., IV UFH).
- Rivaroxaban has similar efficacy and safety compared to therapy with subcutaneous enoxaparin and an oral vitamin K antagonist.25,26
- After 6 to 12 months of standard VTE treatment, continued treatment with rivaroxaban 20 mg PO daily compared to placebo for 6 to 12 additional months decreased the annual risk of symptomatic VTE recurrence by 82%, with a modest increase in hemorrhage.25
- Rivaroxaban’s predominant renal elimination led to exclusion of patients with estimated CrCl <30 mL/minute from VTE treatment trials.
- Rivaroxaban is a substrate of the efflux transporter P-glycoprotein (Pgp), so concurrent use with drugs that interact with Pgp may lead to greater (e.g., amiodarone) or lesser (e.g., St. John’s wort) rivaroxaban exposure.
Apixaban
- Apixaban has recently been approved by the FDA for VTE treatment (10 mg PO bid × 7 days, then 5 mg PO bid).
- Apixaban has similar efficacy to standard enoxaparin/warfarin therapy, but causes less bleeding.27
- After 6 to 12 months of standard VTE treatment, extended-duration treatment (12-month duration) with apixaban at a dose of either 5 mg or 2.5 mg twice daily decreased the annual risk of symptomatic VTE recurrence by about 80%, with a minimal increase in hemorrhage.28
- Apixaban has limited renal elimination but treatment trials excluded patients with an estimated CrCl <25 mL/minute.
Oral Direct Thrombin Inhibitors
Dabigatran
- Compared with warfarin, dabigatran has a more rapid onset, shorter half-life, wider therapeutic window, and more predictable pharmacokinetics.
- In general, dabigatran’s features allow for oral anticoagulant therapy with fixed dosing without INR monitoring, though dabigatran may increase the INR, aPTT, and thrombin time in a nonlinear fashion.
- Compared with warfarin, studies suggest that dabigatran has a lower risk of intracranial (but not gastrointestinal) hemorrhage but a higher risk of myocardial infarction.29–31
- Dabigatran does not have FDA approval for VTE treatment in the United States, though it has approval in other countries (150 mg PO bid after LMWH or UFH for 5 to 11 days).
- After 6 to 18 months of standard VTE treatment, continued treatment with dabigatran (150 mg PO bid) compared to placebo for 6 additional months decreased the risk of VTE recurrence by 92%, with a modest increase in hemorrhage.30
- Dabigatran’s highly predominant renal elimination led to exclusion of patients with estimated CrCl <30 mL/minute from dabigatran trials.
- Dabigatran is not a substrate, inhibitor, or inducer of CYP3A4. However, dabigatran is a substrate of the efflux transporter Pgp, and concurrent use with drugs that interact with Pgp leads to greater (e.g., dronedarone or amiodarone) or lesser (e.g., St. John’s wort) dabigatran exposure.
Warfarin
- The oral anticoagulant warfarin inhibits reduction of vitamin K to its active form and leads to depletion of the vitamin K–dependent clotting factors II, VII, IX, and X and proteins C, S, and Z (see Fig. 12-1).
- Although warfarin has good oral absorption, it requires 4 to 5 days to achieve full anticoagulant effect.
- The initial INR rise primarily reflects warfarin-related depletion of factor VII; the depletion of factor II takes several days due to its relatively long half-life.
- Because of the warfarin-related rapid depletion of the anticoagulant protein C and a slower onset of its anticoagulant effect, patients may develop increased hypercoagulability during the first few days of warfarin therapy if warfarin is not combined with a parenteral anticoagulant.32
- The initial INR rise primarily reflects warfarin-related depletion of factor VII; the depletion of factor II takes several days due to its relatively long half-life.
- The starting dose of warfarin depends upon factors such as age, size, concomitant drug use, and polymorphisms.33 The starting dose ranges from approximately 3 mg in older or petite patients to 10 mg in young, robust outpatients. Patients with polymorphisms in genes for CYP2C9 or vitamin K epoxide reductase (VKORC1) may benefit from cautious low-dose warfarin initiation.34
- For most indications, such as VTE, warfarin has a target INR of 2.5 and a therapeutic range of 2 to 3, though patients with mitral mechanical heart valves should receive a higher level of anticoagulation (e.g., INR target range, 2.5 to 3.5). See Table 14-2.
- Treatment of DVT/PE with warfarin requires overlap therapy with a faster acting anticoagulant (UFH, LMWH, pentasaccharide, or rivaroxaban) for at least 4.5 days and until patients achieve an INR of at least 2.0 for 2 days.35,36
- Warfarin nomogram dosing has more success than does nonstandardized dosing.
- INR monitoring should occur frequently during the first month of therapy (e.g., twice weekly for 1 to 2 weeks, then weekly for 2 weeks, and then less frequently). Typical dose adjustments after the first few weeks of therapy change the weekly dose by 10% to 25%. Subsequent dose adjustments should be smaller, with no dose adjustments needed for INRs that are in the therapeutic range (2 to 3).
- Patients receiving a stable warfarin dose often have INR monitoring monthly. However, patients with labile INRs should have more frequent monitoring (e.g., weekly). Selected, stable patients can have INRs monitored every 6 to 12 weeks.37
- The addition or discontinuation of medications that affect warfarin, especially antifungal agents or sulfa antibiotics, should trigger more frequent INR monitoring and may require dose adjustments of >25%.
- In eligible patients, home monitoring on point-of-care devices can decrease adverse events.38
- Compliant patients who have unacceptable INR lability, or those with LA and an elevated baseline INR, may benefit from long-term anticoagulation with an agent other than warfarin.
- Patients receiving a stable warfarin dose often have INR monitoring monthly. However, patients with labile INRs should have more frequent monitoring (e.g., weekly). Selected, stable patients can have INRs monitored every 6 to 12 weeks.37
TABLE 14-2 Anticoagulation with Artificial Heart Valves
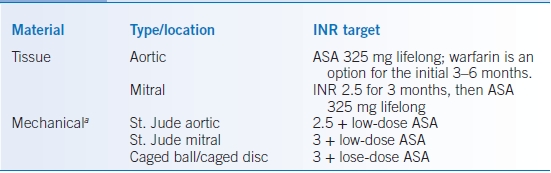
aAdd ASA for any caged valve, known or suspected coronary artery disease, h/o prior stroke, or mitral valve repair.
ASA, acetylsalicylic acid; INR, international normalized ratio.
Other Treatments
- Select patients may benefit from thrombolytic therapy, catheter embolectomy, and emergency surgical thrombectomy, but these are inpatient issues.35,36
- Inferior vena cava (IVC) filters are used for acute VTE when there are absolute contraindications to anticoagulation (e.g., active bleeding, severe thrombocytopenia, or urgent surgery) or recurrent PE despite therapeutic anticoagulation.
- Although prophylactic IVC filters in anticoagulated patients with acute DVT/PE reduce the risk of recurrent PE, a reduction in overall mortality has not been demonstrated, and they increase DVT recurrence.39,40
- Relative indications for IVC filters include primary or metastatic CNS cancer or limited cardiopulmonary reserve after a PE.
- Patients who had an IVC filter placed because of a temporary contraindication to anticoagulation should receive standard-duration anticoagulation when safe (to reduce the risk of filter-related thromboses and recurrent VTE).35,36
- Several types of removable IVC filters exist and can provide a temporary barrier against emboli from the lower extremities, but filter removal requires a second procedure.
- Although prophylactic IVC filters in anticoagulated patients with acute DVT/PE reduce the risk of recurrent PE, a reduction in overall mortality has not been demonstrated, and they increase DVT recurrence.39,40
- Leg elevation reduces edema associated with DVT.
- Ambulation is encouraged for patients with DVT, especially after improvement of pain and edema, though strenuous lower extremity activity should initially be avoided.
- Fitted graduated compression stockings help to reduce the high incidence of postphlebitic syndrome in patients with lower extremity DVT.
- Superficial vein thrombosis (SVT)
- Oral NSAIDs and warm compresses can relieve discomfort.
- For infusion thrombophlebitis (superficial thrombophlebitis associated with a peripheral IV), anticoagulant therapy generally is not used.35
- For patients with spontaneous SVT, treatment with low-dose LMWH (e.g., enoxaparin 40 mg SQ qd) for 8 to 12 days or fondaparinux 2.5 mg PO qd for 45 days may lower the short-term incidence of additional thrombosis.36,41
- Extensive SVT can be treated with a prophylactic dose of fondaparinux for about 6 weeks.35,36
- Recurrent SVT may be treated with anticoagulation.
- Surgical therapy (with ligation of the saphenofemoral junction or stripping of thrombosed superficial veins) of SVT appears to be associated with higher rates of VTE than treatment with anticoagulants.35,36
- Oral NSAIDs and warm compresses can relieve discomfort.
- Catheter-associated upper extremity DVT does not necessarily require catheter removal if it is functional.35
Duration of Anticoagulation
- Duration of anticoagulation depends on patient preferences and values and the risk of recurrent VTE off anticoagulant therapy versus the added risk of bleeding complications from continued anticoagulation.35,36
- Patients with a first episode of VTE provoked by surgical or nonsurgical transient risk factors have a low risk of recurrence (<6%/year) after completing 3 months of anticoagulation.35,36
- Three months of anticoagulant therapy is also sufficient for patients with a first episode of idiopathic VTE associated with other transient risk factors, such as prolonged travel, minor injury, or oral contraceptive pills/hormone replacement therapy that has been stopped.35,36
- For patients with unprovoked proximal lower extremity DVT or PE, at least 3 months of anticoagulation should be prescribed.35,36 Longer (extended) duration of anticoagulant therapy with warfarin (target INR of 2 to 3 or 1.5 to 2), apixaban (either 5 or 2.5 mg, twice daily), rivaroxaban (20 mg PO q day), or dabigatran (150 mg PO bid) reduces the relative risk of recurrent VTE, but it increases the risk of bleeding.28,30
- Patients with cancer and VTE should receive anticoagulation for more than 3 months and possibly until cancer resolution or development of a contraindication.35,36
- For patients with a first VTE and one inherited hypercoagulable risk factor, duration of anticoagulation depends on the type of thrombophilia.36
- Heterozygous factor V Leiden and heterozygous prothrombin 20210A do not necessitate long-term therapy because they increase the odds of VTE recurrence modestly (approximately 1.5-fold).42
- Deficiency of protein S, protein C, or antithrombin carries a high risk of recurrence, which necessitates long-term anticoagulation.42
- Heterozygous factor V Leiden and heterozygous prothrombin 20210A do not necessitate long-term therapy because they increase the odds of VTE recurrence modestly (approximately 1.5-fold).42
- Patients with a first VTE and antiphospholipid antibodies or two inherited risk factors should receive a longer course of anticoagulation (e.g., 12 months), and indefinite therapy should be considered.
- Patients with isolated calf DVT or upper extremity DVT should typically receive standard-duration (e.g., 3 months) anticoagulation.35,36
- Patients with recurrent idiopathic VTE should receive extended-duration (more than 3 months) anticoagulation, possibly indefinitely, unless a contraindication develops, or patient preferences dictate otherwise.35,36
- Patients with a history of VTE, especially those with ongoing risk factors, should possibly receive temporary prophylactic anticoagulation during periods of increased VTE risk (e.g., prolonged air travel).36
- After completing the course of anticoagulant therapy, aspirin (e.g., 81 mg) use should be strongly considered to decrease the risk of recurrent VTE and acute coronary syndrome.43
SPECIAL CONSIDERATIONS
- Outpatient VTE therapy
- Patients selected for outpatient DVT therapy should have no other indications for hospitalization (i.e., other problems or complications of VTE), adequate cardiopulmonary reserve, adequate instruction and understanding of the signs of bleeding and VTE recurrence, access to a telephone, ability to receive the anticoagulant, and adequate outpatient follow-up.44
- Patients selected for outpatient PE therapy should have a low-risk classification and should meet eligibility criteria similar to those used for outpatient DVT therapy.45
- Pregnant women with VTE (and without artificial heart valves) may undergo long-term anticoagulation with SC LMWH, fondaparinux, or UFH, and those who receive LMWH or fondaparinux should undergo factor Xa level monitoring.46
- Pregnant women with VTE should avoid warfarin and the oral thrombin and factor Xa inhibitors. Warfarin is contraindicated in the first trimester of pregnancy because of its teratogenicity, and it is often avoided later in pregnancy because of the risk of fetal bleeding, but it is safe for infants of nursing mothers.
- Patients selected for outpatient DVT therapy should have no other indications for hospitalization (i.e., other problems or complications of VTE), adequate cardiopulmonary reserve, adequate instruction and understanding of the signs of bleeding and VTE recurrence, access to a telephone, ability to receive the anticoagulant, and adequate outpatient follow-up.44
- For failure of oral warfarin or other vitamin K antagonists, with confirmed new VTE despite consistently therapeutic INRs, consider prescribing a different anticoagulant.
COMPLICATIONS/RISK MANAGEMENT
Bleeding
- Major bleeding occurs in 2% to 3% of patients who receive short-term anticoagulant therapy for VTE. The risk of intracranial hemorrhage is probably lower with oral thrombin and factor Xa inhibitors than with vitamin K antagonists. Antiplatelet agents used concomitantly with any anticoagulant nearly double the risk of bleeding.
- Major bleeding in a patient with an acute VTE should lead to the discontinuation of anticoagulation.35,36
- Asymptomatic INR elevation on warfarin
- Asymptomatic minor INR elevations of >3.4 and <5 should be managed by holding or reducing warfarin dose until the INR falls to a safe level and then resuming warfarin at a lower dose.
- Moderate (INR ≥5 but <9) elevation of the INR in asymptomatic patients should be treated by holding one or more warfarin doses. Treatment with oral vitamin K1 1 to 5 mg probably does not reduce the risk of hemorrhage in this setting (as compared with warfarin cessation alone) but lowers the INR (Table 14-3).47
- Severe (INR >9) elevation of the INR should be treated with vitamin K (e.g., oral vitamin K1 2 to 10 mg) unless the INR is likely to be spurious (Table 14-3).48
- Asymptomatic minor INR elevations of >3.4 and <5 should be managed by holding or reducing warfarin dose until the INR falls to a safe level and then resuming warfarin at a lower dose.
- General approach to bleeding with anticoagulants
- Stop the anticoagulant and other drugs (e.g., antiplatelet agents) that may exacerbate bleeding.
- Provide supportive interventions (e.g., IV fluid, compression of bleeding site, surgery).
- Check coagulation tests to see effect of the drug on board, follow CBC, and check CMP.
- Red blood cell transfusion for anemia and platelet transfusion for patients on antiplatelet agents as needed.
- Stop the anticoagulant and other drugs (e.g., antiplatelet agents) that may exacerbate bleeding.
- Anticoagulant-specific approaches may also be necessary.
- Bleeding with warfarin (Table 14-3)49:
- Prothrombin complex concentrate (PCC), or fresh frozen plasma (FFP) if PCC not available, should be given to treat major bleeding associated with warfarin therapy.50,51
- Vitamin K (e.g., 10 mg) by slow IV infusion should be given for serious hemorrhages caused by a high INR. Because of the long half-life of warfarin (approximately 36 hours, depending on CYP2C9 genotype), vitamin K should be repeated every 8 to 12 hours to prevent INR rebound.
- Prothrombin complex concentrate (PCC), or fresh frozen plasma (FFP) if PCC not available, should be given to treat major bleeding associated with warfarin therapy.50,51
- Bleeding with UFH
- Stopping UFH usually restores hemostasis within a few hours.
- With moderate-to-severe bleeding, give FFP.
- For patients receiving UFH who develop major bleeding or have an UFH overdose, UFH can be completely reversed by infusion of protamine sulfate in situations where the potential benefits outweigh the risks (e.g., intracranial bleed, epidural hematoma, and retinal bleed).
- After IV administration, UFH serum concentrations decline rapidly because of a short half-life, so only the UFH administered in the last 2 to 3 hours needs to be reversed with protamine sulfate.
- Approximately 1–mg protamine sulfate IV neutralizes 100 U of UFH, up to a maximum dose of 250 mg.
- Stopping UFH usually restores hemostasis within a few hours.
- Bleeding with LMWH
- With moderate-to-severe bleeding, give FFP.
- For major bleeding associated with LWMH, protamine sulfate has less efficacy compared with its effect on UFH since it neutralizes only approximately 60% of LMWH.52
- With moderate-to-severe bleeding, give FFP.
- Bleeding with fondaparinux
- With moderate-to-severe bleeding, give FFP.
- For patients with very serious bleeding receiving fondaparinux, give concentrated factor VIIa (up to 90 mcg/kg) and tranexamic acid (1-mg IV), but these agents can cause serious thrombosis.53
- With moderate-to-severe bleeding, give FFP.
- Bleeding with oral direct thrombin inhibitor (dabigatran)54
- An increase in the thrombin time (and INR and aPTT) may indicate dabigatran ingestion, but the coagulation tests do not indicate the degree of anticoagulation.
- Determine amount consumed and timing of the last dose. Consider activated charcoal within 2 hours of last dose.
- Consider dialysis for severe bleeding or overdose (especially with renal failure).
- Consider using PCC for severe bleeding (limited data).
- An increase in the thrombin time (and INR and aPTT) may indicate dabigatran ingestion, but the coagulation tests do not indicate the degree of anticoagulation.
- Bleeding with oral factor Xa antagonists54
- An increase in the INR or aPTT may indicate a factor Xa antagonist ingestion, though the coagulation tests do not indicate the degree of anticoagulation.
- Consider using PCC for severe bleeding (limited data).
TABLE 14-3 Treatment of Elevated INR >5
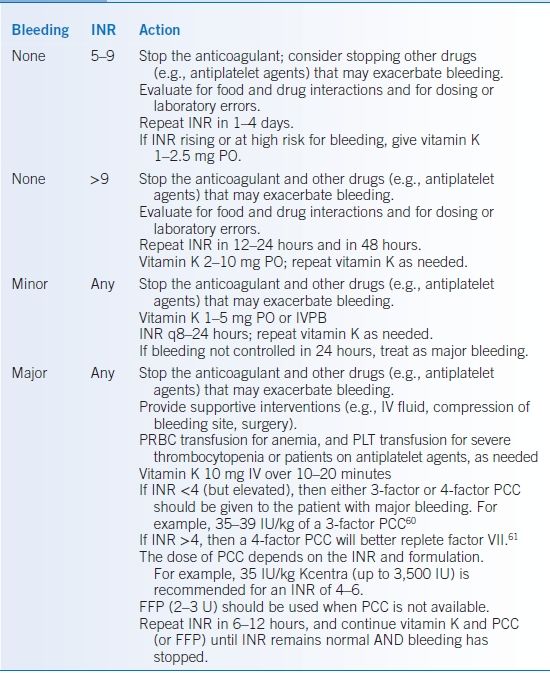
FFP, fresh frozen plasma; INR, international normalized ratio; PCC, prothrombin complex concentrate; PLT, platelets; PRBC, packed red blood cells.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


