Vascular Anomalies of Infancy and Childhood
John B. Mulliken
Arin K. Greene
Vascular anomalies is a newly emerging field involving several medical and surgical specialties. Vascular anomalies all look quite similar, in varying shades of red, pink, and blue. The field has been handicapped by its own confusing clinical and histopathologic terminology. The traditional diagnostic terms failed to guide management. The word “hemangioma” is the most egregious example; it has been used in a generic sense for any type of vascular lesion.
A biological classification of vascular anomalies, first proposed in 1982, was based on clinical findings, natural history, and cellular characteristics. This binary scheme was accepted by the International Society for the Study of Vascular Anomalies (ISSVA) in 1996. Vascular anomalies are broadly divided into two groups: tumors and malformations (Table 1). Vascular tumors are characterized by endothelial cell proliferation. Vascular malformations arise by dysmorphogenesis and have normal endothelial cell turnover. Based on this classification, vascular anomalies can be diagnosed by history and physical examination in 90% of patients. Ten percent of patients require radiographic studies for diagnostic confirmation; histopathology is rarely necessary.
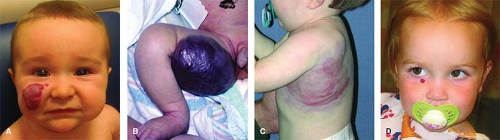
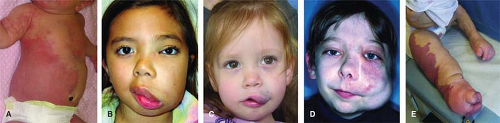
The most common vascular tumors are infantile hemangioma (IH), congenital hemangioma (rapidly involuting congenital hemangioma (RICH), noninvoluting congenital hemangioma (NICH)), kaposiform hemangioendothelioma (KHE), and pyogenic granuloma (PG) (Fig. 1). Malformations are divided into slow-flow lesions (capillary malformation (CM), venous malformation (VM), lymphatic malformation (LM)) and fast-flow lesions (arterial malformations (aneurysm, ectasia, stenosis, fistulas) or arteriovenous malformation (AVM)) (Fig. 2). There are also combined vascular anomalies, often eponymous because of the physician credited for the initial description. One example of a combined vascular anomaly is Klippel–Trenaunay syndrome (KTS), a capillary–lymphatic–venous malformation (CLVM) associated with soft-tissue and skeletal hypertrophy.
Patients with a vascular anomaly often wander from one specialist to another. Their problem seems to fall outside the purview of the general surgeon and vascular surgeon. Since most vascular anomalies present in the skin, these “medical nomads” are usually seen by a dermatologist or plastic surgeon, and sometimes an oncologist because their lesion is considered to be some kind of tumor.
The biological separation of the two major categories, tumors and malformations, has stimulated the formation of vascular anomalies centers in the major referring hospitals. It is clear that no one specialist can have sufficient knowledge to care for these patients. The field of vascular anomalies insinuates among all the surgical specialties, many of the medical disciplines, as well as interventional radiology and pathology. Molecular geneticists are also involved. Causative genes for inheritable lesions have been discovered and many of these syndromes involve vascular lesions of the hollow and solid viscera.
This chapter was written as a primer to encourage general surgeons to adopt the modern terminology of vascular lesions. Surgeons who are curious and fascinated by these common and often insoluble disorders are encouraged to join a vascular anomalies team.
Table 1 Biological Classification of Vascular Anomalies | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||
Vascular Tumors
Infantile Hemangioma
Clinical Presentation
IH is a benign endothelial tumor that occurs in approximately 4% to 5% of Caucasian infants. The old terms “capillary,” “cavernous,” and “strawberry” hemangioma are imprecise and no longer used. IH is more frequent in premature children and in females (4:1). IH typically is single (80%) and involves the head and neck (60%), trunk (25%), or extremity (15%). The median age of appearance is 2 weeks; 30% to 50% of lesions are noted at birth as a telangiectatic stain or ecchymotic area. IH grows faster than the child during the first 9 months of age (proliferating phase). When IH involves the superficial dermis it appears red. A lesion beneath the skin may not be appreciated until 3 to 4 months of age when it has grown large enough to cause a visible deformity; the overlying skin may appear bluish. By age 9 to 12 months, growth of IH reaches a plateau. After 12 months of age the tumor begins to regress (involuting phase), the color fades, and the lesion flattens. Involution ceases in approximately 50% of children by age 5 years (involuted phase). After involution, one-half of children will have an abnormality: residual telangiectasias, scarring, fibrofatty residuum, redundant skin, or destroyed anatomical structures.
Head and Neck Hemangiomas
The majority of IHs are small, harmless lesions that can be monitored under the watchful eye of a pediatrician. Ten percent of proliferating IHs, however, cause significant deformity or complications, usually when located on the head or neck. Ulcerated lesions may destroy the eyelid, ear, nose, or lip. IH of the scalp or eyebrow can result in alopecia. Periorbital hemangioma can block the visual axis or distort the cornea, causing amblyopia. Subglottic hemangioma may obstruct the airway.
Multiple Hemangiomas
Approximately 20% of infants have more than one IH. The term hemangiomatosis designates five or more small (<5 mm) tumors. These children are at increased risk for IH of internal organs, although the risk is low. The liver is most commonly affected; the brain, gut, or lung are rarely involved. Ultrasonogram should be considered to rule out hepatic IH.
Hepatic Hemangiomas
The liver is the most common extracutaneous site for IH. Ninety percent of fast-flow hepatic lesions are IH. The differential diagnosis includes AVM, hepatoblastoma, and metastatic neuroblastoma, which do not demonstrate significant shunting on imaging. There are three subtypes of hepatic hemangioma: focal, multifocal, and diffuse.
Although most hepatic IHs are nonproblematic and discovered incidentally, large tumors can cause heart failure, hepatomegaly, anemia, or hypothyroidism. Focal hepatic hemangioma usually are asymptomatic and not associated with cutaneous lesions; they are often identified prenatally. There is evidence that solitary hepatic hemangioma is a RICH. Occasionally this tumor can cause cardiac overload and thrombocytopenia; however, these symptoms resolve as the tumor regresses. Multifocal hepatic IHs are often accompanied by cutaneous lesions. Although usually asymptomatic, multifocal lesions can cause high-output cardiac failure, which is managed by corticosteroid or embolization. Diffuse hepatic IH can cause massive hepatomegaly, respiratory compromise, or abdominal compartment syndrome. Infants are also at risk for hypothyroidism and irreversible brain injury because the large tumor volume expresses enough deiodinase to inactivate thyroid hormone. Patients require thyroid stimulating hormone monitoring and, if abnormal, intravenous thyroid hormone replacement until the IH begins to regress.
Although most hepatic IHs are nonproblematic and discovered incidentally, large tumors can cause heart failure, hepatomegaly, anemia, or hypothyroidism. Focal hepatic hemangioma usually are asymptomatic and not associated with cutaneous lesions; they are often identified prenatally. There is evidence that solitary hepatic hemangioma is a RICH. Occasionally this tumor can cause cardiac overload and thrombocytopenia; however, these symptoms resolve as the tumor regresses. Multifocal hepatic IHs are often accompanied by cutaneous lesions. Although usually asymptomatic, multifocal lesions can cause high-output cardiac failure, which is managed by corticosteroid or embolization. Diffuse hepatic IH can cause massive hepatomegaly, respiratory compromise, or abdominal compartment syndrome. Infants are also at risk for hypothyroidism and irreversible brain injury because the large tumor volume expresses enough deiodinase to inactivate thyroid hormone. Patients require thyroid stimulating hormone monitoring and, if abnormal, intravenous thyroid hormone replacement until the IH begins to regress.
Hemangiomas and Structural Anomalies
There are uncommon presentations of IH with malformations, either in the head/neck or in the lumbosacral regions. PHACE association affects 2.3% of patients with IH, and consists of a plaque-like IH in a regional distribution of the face with at least one of the following anomalies: Posterior fossa brain malformation; Hemangioma; Arterial cerebrovascular anomalies; Coarctation of the aorta and cardiac defects; Eye/Endocrine abnormalities. When ventral developmental defects (Sternal clefting or Supraumbilical raphe) are present, an “S” is added (PHACES). Ninety percent of infants are female and cerebrovascular anomalies are the most common associated finding (72%). Because 8% of children with PHACE have a stroke in infancy, patients should have magnetic resonance imaging (MRI) to evaluate the brain and cerebrovasculature. Infants are referred for ophthalmologic, endocrine, and cardiac evaluation to rule out these associated anomalies.
Reticular hemangioma is an uncommon variant of IH that most commonly affects the lumbosacral area and lower extremity. Females (83%) are usually affected. Unlike typical IH, reticular tumors are likely to ulcerate and rarely cause cardiac overload. Reticular hemangioma is often associated with ventral–caudal malformations (omphalocele, recto-vaginal fistula, vaginal/uterine duplication, solitary/duplex kidney, imperforate anus, tethered cord lipomyelomeningocele). After involution small veins often remain, which may be treated by sclerotherapy. Ultrasonography (US) is obtained to rule out associated anomalies in infants less than 4 months of age. MRI is indicated in older infants or when US is equivocal.
Diagnosis
Most IHs are easily diagnosed by history and physical examination. Fast flow is confirmed using a hand-held Doppler device. By formal US, IH appears as a soft-tissue mass with fast flow, decreased arterial resistance, and increased venous drainage. On MRI the tumor is isointense on T1, hyperintense on T2, and enhances during the proliferating phase. Involuting IH has increased lobularity and adipose tissue; the number of vessels and flow is reduced. Rarely, biopsy is indicated if malignancy is suspected or if the diagnosis remains unclear following imaging
studies. Positive erythrocyte-type glucose transporter (GLUT 1) immunostaining differentiates IH from other vascular tumors and malformations.
studies. Positive erythrocyte-type glucose transporter (GLUT 1) immunostaining differentiates IH from other vascular tumors and malformations.
Nonoperative Treatment
Most IHs are simply observed because 90% are small, localized, and do not involve anatomically important areas. During the proliferative phase 16% of lesions will ulcerate; most commonly on the lips, neck, and anogenital region. Other complications include bleeding and infection. IH is kept moist during the proliferative phase with hydrated petroleum to minimize desiccation as well as to protect against incidental trauma. IH may be further protected by using a petroleum gauze barrier. If an ulceration develops it is managed with local wound care; often healing takes 4 to 6 weeks.
Topical Corticosteroid
Topical corticosteroid has minimal efficacy; especially against IH involving the deep dermis and subcutis. Ultrapotent agents may be effective for a very superficial IH. Although lightening may occur, if there is deep component it will not be affected. Adverse effects include hypopigmentation, skin atrophy, and even adrenal suppression.
Intralesional Corticosteroid
Small, well-localized IHs that obstruct the visual axis or nasal airway or those at risk for damaging important structures (e.g., eyelid, lip, nose) are best managed by intralesional corticosteroid. Triamcinolone (3 mg/kg) stabilizes the growth of the lesion in at least 95% of patients; 75% of tumors will decrease in size. The corticosteroid lasts 4 to 6 weeks and thus infants may require one to two more injections during the proliferative phase. Intralesional corticosteroid may cause subcutaneous fat atrophy. Blindness has been reported following injection of deep periorbital hemangioma due to embolic occlusion of the retinal artery.
Systemic Corticosteroid
Any problematic IH that is larger than 3 to 4 cm in diameter is managed by daily oral prednisolone. The patient is started on 3 mg/kg/d for 1 month, which then is tapered by 0.5 cc every 2 to 4 weeks until it is discontinued between 10 and 12 months of age when the tumor is no longer proliferating. Recently, propranolol has been described for the treatment of IH, but its efficacy and safety, compared with corticosteroid, has not been studied. Corticosteroid, in contrast, has been used to treat IH for over 40 years and has proven to be very safe and effective. Meta-analysis has shown that 84% of patients treated with different doses of corticosteroid will have (a) stabilization of growth or (b) accelerated regression. Almost all patients, however, will respond to 3 mg/kg. Treatment response is usually evident within 1 week of therapy by signs of involution: decreased growth rate, fading color, and softening of the lesion. The location of the hemangioma does not influence response rate. For the rare lesion that fails to stabilize with corticosteroid, the dose may be increased up to 5 mg/kg, which may improve treatment response. Alternatively, the child may be switched to vincristine. Interferon is no longer recommended in children less than 12 months of age because it can cause neurologic sequela, particularly spastic diplegia.
Complications of systemic corticosteroid for the management of IH have been studied; there are no adverse effects on neurodevelopment. Short-term morbidity may include cushingoid face, personality change, gastric irritation, fungal (oral or perineal) infection, myopathy, decreased gain in height, and decreased gain in weight. These findings resolve after the completion of therapy. Over 90% of children return to their pretreatment growth curve for height by 24 months of age.
Embolic Therapy
Large IHs, most commonly multifocal hepatic lesions, can cause high-output congestive heart failure. Embolization may be indicated for the initial control of cardiac overload while systemic corticosteroid therapy takes effect. Cardiac failure often recurs even after initial improvement, and drug therapy should be continued after embolization until the child is approximately 12 months of age when natural involution begins.
Laser Therapy
There is little, if any, role for pulsed-dye laser treatment for proliferating IH. The laser penetrates only 0.75 to 1.2 mm into the dermis, and thus only affects the superficial portion of the tumor. Although lightening may occur, the mass of IH is not affected. Instead, patients have an increased risk of skin atrophy and hypopigmentation. The thermal injury delivered by the laser to the ischemic dermis increases the risk of ulceration, pain, bleeding, and scarring. Pulsed-dye laser is indicated during the involuted phase to fade residual telangiectasias.
Operative Treatment
Proliferative Phase (Infancy)
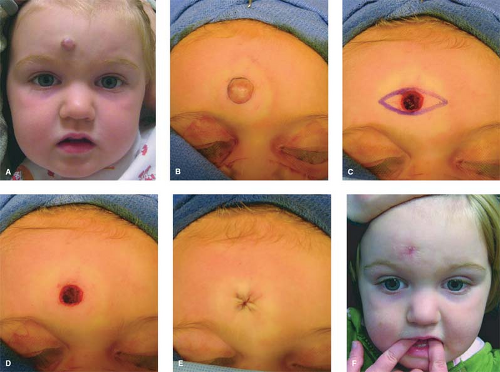
Operative treatment in infancy is generally not recommended. The tumor is highly vascular during this period and there is a risk for blood loss, iatrogenic injury, and an inferior aesthetic outcome, compared with excising residual tissue after the tumor has regressed. Nevertheless, in experienced surgical hands, there are indications for operative intervention during the proliferating phase: (a) failure or contraindication to corticosteroid; (b) well-localized tumor in an anatomically favorable area; (c) resection will be necessary in the future and the scar would be the same. Circular lesions located in visible areas, particularly the face, are best removed by circular excision and purse-string closure. This technique minimizes the length of the scar as well as distortion of surrounding structures. Lenticular excision of a circular hemangioma results in a scar as long as three times the diameter of the lesion (Fig. 3). In comparison, a two-stage circular resection followed by lenticular excision/linear closure 6 to 12 months later will leave a scar approximately the same length as the diameter of the original hemangioma (Fig. 4).
Involuting Phase (Early Childhood)
While operative management of IH is generally not indicated during the proliferative phase, resection during involution is much safer because the lesion is less vascular and smaller. Because the extent of the excision is reduced, the outcome is superior. Approximately 50% of IHs leave behind fibrofatty tissue or damaged skin after the tumor regresses, causing a deformity. Sometimes a child requires reconstruction of damaged structures (e.g., nose, ear, lip). Staged or total excision should be considered during this period, rather than waiting for complete involution if (a) it is clear that the lesion will require resection (e.g., postulceration scarring, destroyed structures, expanded skin, significant fibrofatty residuum); (b) the length of the scar would be similar if the procedure was postponed to the involuted phase; (c) the scar is in a favorable location. Advantages of operative intervention during this period, compared with late childhood, is that reconstruction is under way prior to the child’s development of memory or awareness of a facial difference.
Involuted Phase (Late Childhood)
Waiting until IH has fully involuted prior to resection ensures that the least amount of fibrofatty residuum and excess skin is resected, resulting in the smallest possible scar. Postponing intervention until complete involution has occurred must be weighed against the possible psychosocial implications of maintaining a deformity until late childhood. Allowing for full involution is recommended for lesions when it is unclear if a surgical scar would leave a worse deformity than the appearance of the residual hemangioma.
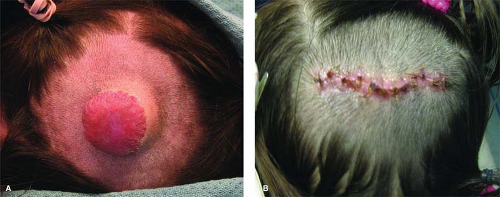 Fig. 3. A 2-year-old female with involuting phase IH of the scalp resulting in fibrofatty residuum and alopecia: lenticular excision and linear closure. |
Congenital Hemangiomas
Clinical Presentation
There are rare hemangiomas that arise in the fetus, are fully grown at birth, and do not have postnatal growth. These congenital hemangiomas are red-violaceous with coarse telangiectasias, central pallor, and a peripheral pale halo. These lesions are more common in the extremities, have an equal sex distribution, and are solitary with an average diameter of 5 cm. There are two forms: RICH and NICH. RICH regresses rapidly after birth; 50% have completed regression by 7 months of age. RICH affects the head or neck (42%), limbs (52%), or trunk (6%). RICH does not leave behind a significant adipose component, unlike IH. NICH, in contrast, does not undergo involution; there is persistent fast flow. It involves the head or neck (43%), limbs (38%), or trunk (19%).
Treatment
RICH usually does not require resection in infancy because it undergoes accelerated regression. Occasionally, RICH is complicated by congestive heart failure, which is controlled by corticosteroid or embolization as the lesion involutes. After regression RICH may leave behind atrophic skin and subcutaneous tissue. Reconstruction with autologous grafts (fat, dermis) or acellular dermis may be indicated. NICH is rarely problematic in infancy; it is observed until the diagnosis is clear. Resection of NICH may be indicated to improve the appearance of the affected area, as long as the surgical scar will be less noticeable than the lesion.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree