Uterine Smooth Muscle Tumors
Hemorrhagic Cellular Leiomyoma and Hormone-Induced Changes
Leiomyoma Treated by Interventional Radiology
Leiomyoma with Bizarre Nuclei (So-Called ‘Atypical’ Leiomyoma)
Unusual Growth Patterns of Leiomyomas
Atypical Smooth Muscle Tumors (So-Called Smooth Muscle Tumors of Uncertain Malignant Potential)
Introduction
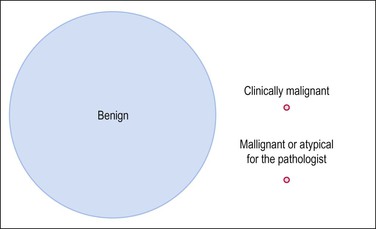
Smooth muscle tumors of the uterus are very common and the vast majority are benign leiomyomas. In contrast, leiomyosarcomas are rare and constitute only 1.3% of uterine malignancies (Figure 19.1). Nevertheless, leiomyosarcoma is the most frequent malignant mesenchymal tumor of the uterus, accounting for almost 60% of uterine sarcomas. Whereas almost all leiomyosarcomas are high-grade tumors and their diagnosis is straightforward, a small fraction of uterine smooth muscle tumors show atypical histologic features that are insufficient for the diagnosis of malignancy or have an unpredictable clinical behavior. The term smooth muscle tumors of uncertain malignant potential (STUMP) has been used to describe these neoplasms; however, we prefer to call them atypical smooth muscle tumors in view of their favorable behavior in most cases. The latter term simply describes the morphologic findings avoiding the words ‘uncertain’ and ‘malignant,’ which undoubtedly create unnecessary concern for the patient. A classification of smooth muscle tumors of the uterus is given in Table 19.1.
Leiomyoma
Incidence
Leiomyomas are clinically evident in 20–30% of women over 30 years of age,1,2 rising to more than 40% in those over 40 years old.3 In a series of 1000 uteri that were serially examined, over 56% contained leiomyomas.4 In other studies, 69–77% of women who underwent hysterectomy for non-cancerous conditions were found to have leiomyomas.5,6 The clinical presentation depends on their size and location.1 Most are asymptomatic while others present abnormal uterine bleeding, pain, and/or abdominal enlargement.
Leiomyomas are more common in black than in Caucasian women.6,7 Recently, a higher prevalence for genetic polymorphisms in the estrogen receptor (ER)-α and catechol-O-methyltransferase (an enzyme involved in estrogen metabolism) was observed in black women with leiomyomas, but it is unknown whether these polymorphisms account for the higher tumor incidence in these patients.8,9 In fact, a number of nuclear receptors are differentially expressed by leiomyomas in black women compared to other ethnic groups.10
Like the contribution of race, familial patterns of inheritance also suggest that genetic risk factors are important in the pathogenesis of leiomyomas.11,12
Etiology
The precise etiology of leiomyomas is unknown, but the hormonal milieu is pivotal.13 Recent evidence confirms that estrogens and progesterone receptors (ERs and PRs) are important in their pathogenesis.14 Studies comparing gene expression in leiomyomas with that in normal myometrium show that leiomyomas maintain a high level of sensitivity to estrogen during the estrogen-dominated proliferative phase of the menstrual cycle.15 Furthermore, cultured cells from leiomyomas have a significantly higher response to estrogen than do matched cultures of myometrial cells from the same patient, particularly if the tissue is taken for culture in the proliferative phase.16 Semiquantitative immunohistochemistry for ERs and PRs correlates with tumor growth rate.17 Accelerated growth, sufficient to require hysterectomy, also occurs in women taking tamoxifen for breast cancer treatment.18,19
Further information on the origin of leiomyomas has come from studying their clonality. Originally, glucose-6-phosphate dehydrogenase isoforms were used as a marker for X chromosome inactivation,20,21 but this has been supplanted by newer molecular biologic techniques that exploit methylation differences between polymorphic loci on the active and inactive X chromosomes.22–24 These methods confirm that each leiomyoma derives from a single transformation event.25 Interestingly, these studies also suggest that each tumor is a distinct clone, reinforcing the notion that smooth muscle tumorigenesis is an exceedingly common event.
The genetic mechanisms by which initiation and growth of leiomyomas occur so frequently are not fully understood. Cytogenetic analysis of these benign smooth muscle tumors, however, has revealed important clues.12 Nearly one-half of leiomyomas have chromosomal rearrangements large enough to be seen in G-banded karyotypes. These chromosomal rearrangements are generally simple, which is in sharp contrast to the aberrations seen in leiomyosarcoma. To date, recurrent aberrations have allowed the definition of seven cytogenetic subgroups: t(12;14)(q14–15;q23–24), del(7)(q22q32), rearrangements of 6p21 and 10q22, trisomy 12, and deletions of 3q and 1p.12,26 Of these, the translocation between chromosomes 12 and 14 and the rearrangements involving chromosome 6 are perhaps the best understood. Both rearrangements involve genes for two closely related non-histone chromatin proteins: HMGA1 at 6p21 and HMGA2 at 12q15.27–29 Rearrangements involving HMGA1 and HMGA2 are also associated with lipomas, endometrial polyps, vulvar aggressive angiomyxoma, and several other benign mesenchymal neoplasms.30,31 Evidence suggests that inappropriate expression of AT-hook DNA binding domains from HMGA2 are relevant in uterine leiomyoma.32,33 Interestingly, rearranged 10q22 disrupts a gene for another class of chromatin protein, namely the histone acetyltransferase MYST4, which raises the possibility that chromatin regulation more broadly is important in the pathogenesis of uterine leiomyoma.34 Beyond such mechanistic insights, cytogenetic studies may also have some practical significance. Tumors with chromosomal rearrangements are on average larger and often within the uterine wall.35 In addition, some aberrations are associated with specific variants of uterine leiomyomas.
An autosomal dominant syndrome, Reed syndrome36 (Mendelian Inheritance in Man #150800 and #605839), exhibits a predisposition toward cutaneous and uterine leiomyomas as well as renal cell carcinoma. This syndrome has inactivated fumarate hydratase,37,38 an enzyme of the Krebs cycle. Germline fumarate hydratase mutations behave like those in classical tumor-suppressor genes. Some non-syndromic (i.e., typical ‘garden variety’) leiomyomas also have a subset, particularly those from symptomatic younger women, which may have deleted fumarate hydratase.11,39,40
Transcriptional profiling has been used to study uterine smooth muscle tumors.33,41,42 While lists of dysregulated genes produced by various groups overlap to some degree, and while much more study is needed to understand fully the significance of these transcriptional profiles, it seems that the transcriptional profiles for uterine leiomyomas are much closer to myometrium than to leiomyosarcoma and atypical leiomyomas, and that malignant transformation appears to coincide in downregulation of gene expression more frequently than upregulation.43 Three potential mechanisms proposed to account for differential gene regulation in the smooth muscle transcriptome include: (1) differential expression of non-histone chromatin proteins such as HMGA2 and MYST4; (2) altered expression of tuberin and the glucocorticoid receptor;10 and (3) abnormal regulation of micro-RNAs, which in turn might regulate HMGA2.44
Gross Features
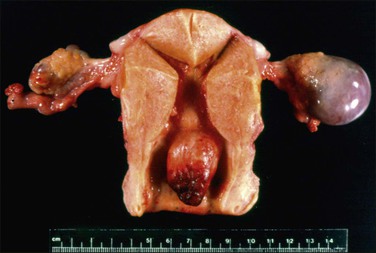
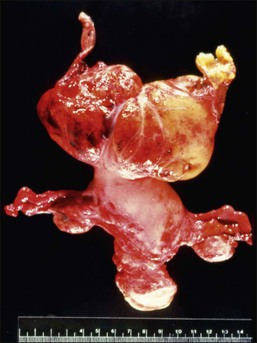
Leiomyomas occur anywhere within the myometrium and are multiple in about two-thirds of cases. They also occur occasionally in the cervix (see Chapter 13). The most frequent location is within the myometrial wall where, if numerous or large, they can also grossly distort the uterus (Figure 19.2). Those situated close to the endometrium or the serosa are referred to as submucosal and subserosal, respectively. Submucosal leiomyomas are frequently ulcerated and may lead to intermenstrual bleeding (Figure 19.3). A subserosal, pedunculated leiomyoma may, on rare occasions, lose its connection with the uterus and become attached to another pelvic structure, such as the omentum, bowel, or peritoneum (‘parasitic’ leiomyoma), which must not be mistaken for a metastasis from a malignant smooth muscle tumor. Leiomyomas are round, firm, and rubbery and they rise above the surrounding myometrium from which they are easily shelled out. The cut surface is typically white to tan, with a whorled, spiral pattern. A striking feature is the very sharp demarcation between it and the surrounding normal myometrium. Several degenerative changes may occur in leiomyomas. Hemorrhage and necrosis are frequent in leiomyomas, particularly if they are large or occur in women who are pregnant or receiving progestogen. Whereas the hemorrhagic areas appear dark, necrotic zones are yellow. Cystic change and calcification also occur.
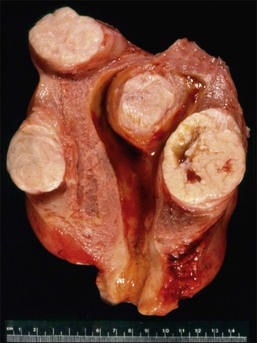
Figure 19.2 Multiple leiomyomas. On cut section, the tumors have a whorled white-tan surface that bulges above the normal myometrium. Three leiomyomas are intramural and one submucosal.
Microscopic Features
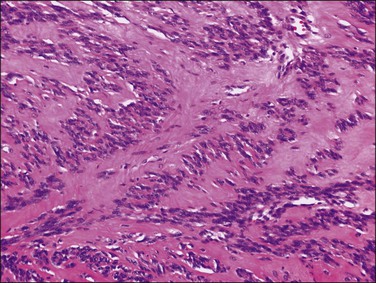
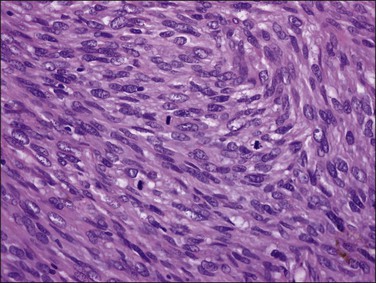
A leiomyoma consists of intersecting bundles of smooth muscle cells. The margins are well circumscribed (Figure 19.4). The smooth muscle cells are markedly elongated and have eosinophilic cytoplasm and tapered, cigar-shaped nuclei. In a typical leiomyoma, the nuclei are uniform and mitotic figures absent or sparse (Figure 19.5). Abundant reticulin is present. The smooth muscle cells of a leiomyoma are usually more closely packed than those of the surrounding myometrium, so that the tumor usually appears more cellular and the small blood vessels appear compressed and less randomly distributed. Such increased cellularity is often particularly striking in women past menopause. The nuclei in a leiomyoma are generally arranged in a fascicular fashion, but occasionally there is palisading resulting in a pattern similar to that seen in a neurilemmoma (schwannoma) (Figure 19.6).
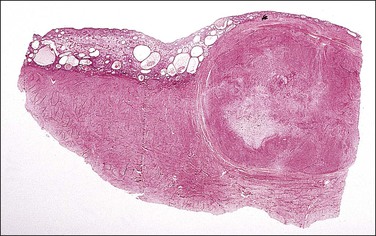
Figure 19.4 Submucosal leiomyoma. At this low magnification the sharp line of demarcation between the leiomyoma and the surrounding myometrium is clearly shown. The overlying endometrium is compressed and atrophic.
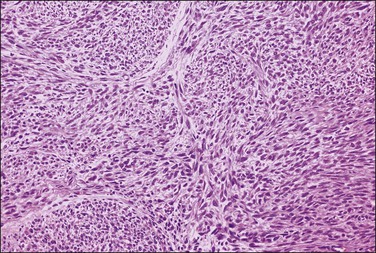
Figure 19.5 Leiomyoma. Intersecting bundles of elongated smooth muscle cells exhibiting eosinophilic cytoplasm and cigar-shaped nuclei. The nuclei are uniform and mitotic figures absent.
Marked attenuation of overlying endometrium may be seen in some mucosal leiomyomas (Figure 19.4). By extension, the presence of aglandular functionalis in curettings is a hint that dysfunctional uterine bleeding might be due to a nearby leiomyoma.
Variants of Leiomyoma
Mitotically Active Leiomyoma
In premenopausal women, otherwise typical leiomyomas may occasionally show 5 or more mitotic figures per 10 HPF. These tumors have a benign clinical course (even when treated by myomectomy).45–47 They are typically small (<10 cm) and have a benign gross appearance. Approximately 60% of mitotically active leiomyomas are submucosal. Microscopically, they have 5–14 mitotic figures per 10 HPF when counted in the most active area. This increased proliferative rate is frequently, but not always, diffusely distributed (Figure 19.7). In submucosal leiomyomas, ulceration, inflammation, or necrosis may be accompanied by a focal increase of proliferation and mitotic activity. Increased proliferation has also been associated with higher progestin levels, such those seen during the secretory phase.48 Malignant tumors exhibiting severe nuclear atypia, abnormal mitoses, or geographic necrosis should not be diagnosed as mitotically active leiomyomas.
Cellular Leiomyoma
A cellular leiomyoma is a benign smooth muscle tumor that has a cellularity greater than the surrounding myometrium and the majority of leiomyomas.49,50 Grossly, cellular leiomyoma may resemble typical leiomyoma but often has a fleshier and softer sectioned surface and the color tends to be tan or creamy yellow rather than pinkish white. Microscopically, cellular leiomyomas almost always have <5 mitotic figures per 10 HPF and are cytologically bland. A fascicular pattern is present in some areas. The tumor is markedly cellular, and the cells are small and round to spindle shaped (Figure 19.8). The blood vessels are typically large with thick muscular walls and cleft-like spaces are often seen, possibly representing compressed vessels or edema.50 Unlike the usual leiomyoma, cellular leiomyomas often show focal extensions into and appear to merge with the adjacent myometrium.
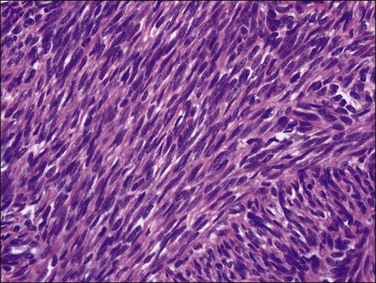
Figure 19.8 Cellular leiomyoma. The nuclear features are the same as those of a typical leiomyoma but the nuclei are more closely packed.
Differential Diagnosis
Cellular leiomyomas may resemble endometrial stromal tumors.50 Helpful features in the differential diagnosis include: (1) coexistence of the highly cellular areas with a fascicular growth pattern typical of smooth muscle tumors; (2) reticulin stains with fibers that tend to parallel the cell bundles in leiomyomas but surround individual tumor cells in endometrial stromal tumors; (3) vessels of large caliber with thick muscular walls, in contrast to the prominent network of small blood vessels typical of endometrial stromal tumors; (4) presence of cleft-like spaces and the absence of foamy histiocytes, which are often present in endometrial stromal tumors; and (5) strong and multifocal or diffuse immunoreactivity for smooth muscle markers such as desmin and h-caldesmon.
Hemorrhagic Cellular Leiomyoma and Hormone-Induced Changes
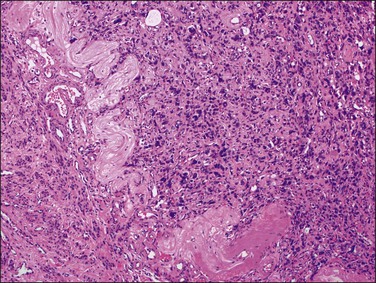
Hemorrhagic cellular leiomyoma, or ‘apoplectic leiomyoma,’ occurs in pregnancy and during treatment with oral contraceptive or gonadotrophin-releasing hormone agonists (GnRHa). Grossly, hemorrhage and cystic change are frequently seen (Figure 19.9).52,53 Microscopically, the leiomyoma is densely cellular and contains stellate zones of recent hemorrhage. Mitotic activity may be increased (up to 8 mitotic figures per 10 HPF), but there is no atypia and necrosis generally is not present. Vascular changes may be prominent. Leiomyomas treated with GnRHa, to reduce their size prior to their removal, may exhibit the features of apoplectic leiomyomas and vascular changes (i.e., myxoid change, fibrinoid change, mural thickening, luminal narrowing, and thrombosis). Leiomyomas removed several weeks after withdrawal of GnRHa treatment may have increased mitotic activity.54
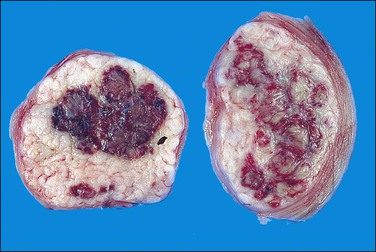
Figure 19.9 After treatment with GnRH agonists, the leiomyoma shows focal areas of hemorrhagic necrosis.
The most striking feature that may be present is geographic (coagulative) necrosis exhibiting nuclear pyknosis, karyorrhexis, karyolysis, and markedly increased cytoplasmic eosinophilia.55 This may affect a small group of cells or extensive areas within the leiomyoma and be surrounded by a rim of inflammatory cells. Apoptosis may be prominent.56 Changes in cellularity are not significant and both decreased57 and increased55 cellularity have been reported. A massive lymphocytic infiltration58 and thickening of blood vessel walls with narrowing of the lumen may also be seen.57 A study of cell proliferation indices (Ki-67 and proliferating cell nuclear antigen) suggests that the reduction in size of leiomyomas treated by GnRHa is due to a reduction in the number of cycling cells, presumably secondary to reduced levels of ERs and PRs.59
Degenerated Leiomyoma
A variety of degenerative changes can occur in leiomyomas. By far the most common form of degeneration is hyaline change whereby expanded septa have lost their fibrillary structure, assuming a uniform, pale eosinophilic, ground-glass appearance (Figure 19.10). This change may be localized or it may affect extensive areas of the tumor, occasionally even the whole of it. This form of degeneration may be accompanied by surviving muscle cells oriented into lacework patterns. The blood vessels within an area of hyaline necrosis undergo the same change and can be seen as pale outlines, a point of distinction from the geographic tumor cell necrosis seen in leiomyosarcoma where the vessels are often spared.60 Degenerated areas may liquefy, resulting in hydropic or cystic degeneration. When extreme, such a degenerated leiomyoma may take on a peculiar multinodular appearance.61 Mucoid and myxoid degeneration are also common. In myxoid change, the scattered nuclei are embedded in an amorphous, slightly amphophilic matrix whereas in mucoid degeneration the matrix appears to be mucinous in nature. The mucoid and myxoid forms of degeneration lack practical importance and the two terms are often used interchangeably.
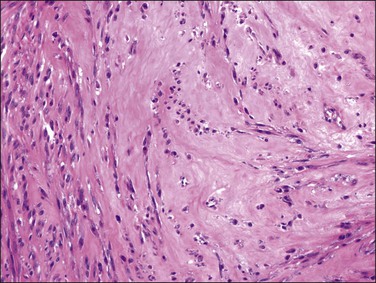
Figure 19.10 Leiomyoma with hyaline change. The tumor shows a uniform, eosinophilic, ground-glass appearance.
Red degeneration (necrobiosis), on the other hand, occurs characteristically but not exclusively in pregnancy and often causes pain and fever. Necrobiosis results in the cut surface taking on a more homogeneous look with loss of the whorled appearance. At the same time, the color becomes a deeper pink or red (due to staining by fresh blood pigment) and the consistency softer (Figure 19.11). Over time, the periphery of a leiomyoma that has undergone red degeneration may become white and calcified. Unlike hyaline change, the microscopic appearance in red degeneration shows the ghosts of the muscle cells and their nuclei. Uncommonly, a leiomyoma may undergo necrosis, resulting in a soft, structureless, pale gray mass. This change is seen most often in submucous leiomyomas that protrude into the endometrial cavity.
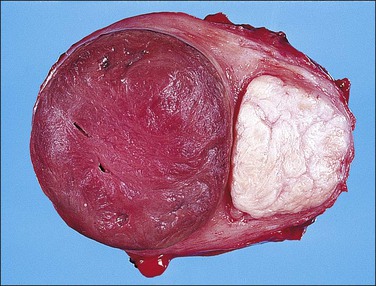
Figure 19.11 Red degeneration (necrobiosis). The cut surface of the leiomyoma on the left is red and more homogeneous, with loss of the whorled appearance.
Calcific degeneration is seen more frequently in women after the menopause.
Leiomyoma Treated by Interventional Radiology
Selective arterial embolization is one such conservative method for treating uterine leiomyomas.62 The complication most interesting to pathologists is the delay in diagnosis of leiomyosarcoma.63–65 In about 80–90% of women who underwent embolization, the symptoms improved sufficiently for surgical treatment to be avoided.62 Gross examination of hysterectomy specimens may sometimes reveal distended small arteries occluded by small aggregates of translucent spheres that might conjure up the notion of a bizarre parasitic infection to the unaware examiner. Such occluded vessels may be found throughout the specimen, and not necessarily in proximity to leiomyomas. Microscopically, a foreign-body giant cell reaction surrounds the amorphous spheroids after the initial period following instillation.66 The leiomyomas themselves may show various patterns of necrosis, including hyaline, coagulative, and suppurative types, or they may show no apparent change at all.66
Another emerging technology for the noninvasive treatment of leiomyoma is ablation by magnetic resonance (MR)-guided focused ultrasound (FUS or MRgFUS).67 Patients are placed in a specialized MR scanner that has been modified to include a large panel of ultrasound transducers. The ultrasound emissions interfere in a small focus and result in very rapid tissue heating and thermal necrosis, which may grossly mimic the geographic tumor necrosis seen in leiomyosarcoma. One clue that distinguishes this treatment effect from malignant-type geographic tumor necrosis is the firmness of the tissue section. Sudden thermal ablation results in massive protein denaturation, resulting in a hard, unyielding cut surface, whereas necrosis in leiomyosarcomas produces additional softening in tissue. Microscopic inspection of the FUS treatment effect is also notable for the sharp transition from viable to nonviable tissue, at least in the short term following treatment. In contrast to leiomyosarcoma, the necrosis following FUS comprises a remarkably bland eosinophilia typical of thermal denaturation.
Leiomyoma with Bizarre Nuclei (So-Called ‘Atypical’ Leiomyoma)
Even if nuclear atypia is necessary for the diagnosis of malignancy in uterine smooth muscle tumors, as an isolated finding, it is insufficient for that purpose. Furthermore, leiomyomas may occasionally show giant cells with pleomorphic nuclei and little or no mitotic activity.68 The terms ‘symplastic leiomyoma,’ ‘bizarre leiomyoma,’ and ‘pleomorphic leiomyoma’ are older synonyms for leiomyomas exhibiting this striking change in the absence of tumor cell necrosis and abnormal mitotic figures. Most occur during reproductive age (mean age 40.7 years). In a series of 24 cases in which most patients were treated by hysterectomy and a minority by myomectomy, no deaths or recurrences were recorded after a prolonged follow-up, underscoring the benign nature of this histologic variant of leiomyoma.69,70 In other words, this peculiar type of nuclear atypia differs from that encountered in leiomyosarcomas; it is not ‘premalignant’ as implied by the term ‘atypia’ in most precancerous lesions, and should be categorized separately.
Grossly, bizarre tumors may resemble conventional leiomyomas or show yellow or tan areas, hemorrhage, softening, cavitation or myxoid change (Figure 19.12).69 In contrast with the large size of most leiomyosarcomas, bizarre leiomyomas are usually small (<5.5 cm).
Microscopically, nuclear atypia is easily appreciated at lower magnification (i.e., with 4 or 10× objectives) in most cases. The pleomorphic cells with abundant eosinophilic cytoplasm and atypical nuclei with prominent pseudoinclusions (invaginations of brightly eosinophilic cytoplasm) may appear unifocal, multifocal, or diffusely distributed (Figure 19.13). Many of these cells are multinucleated or have multilobed nuclei, but large hyperchromatic mononuclear cells are also common. Some of the nuclear features are degenerative, including smudged chromatin, vacuolation, karyorrhexis, and pyknosis; however, most tumors also contain cells with ominous nuclear features such as coarsely clumped or granular chromatin with areas of clearing and enlarged nucleoli;69 yet, in alternate bands of tumor uninvolved by the pleomorphic cells, the spindled smooth muscle cells are uniform and show bland nuclei, as seen in ordinary leiomyomas69 (Figure 19.13A). This ‘zebra-like’ pattern helps in the distinction between bizarre leiomyoma and leiomyosarcoma. Mitotic activity in bizarre leiomyomas is usually low. In the series mentioned earlier,69 the mean mitosis count was only 1.6 mitotic figures per 10 HPF by the highest count method and 0.8 mitotic figures per 10 HPF by the average count method.69 However, one tumor had up to 7 mitotic figures per 10 HPF.47,69,70 Most bizarre leiomyomas have 0–4 mitotic figures per 10 HPF. The mitotic figures are only rarely atypical (e.g., multipolarity or extreme polyploidy). Furthermore, these tumors may show degeneration, edema, and hyaline change, with the bizarre cells typically present at the edge of the degenerating areas and around blood vessels (Figure 19.14).
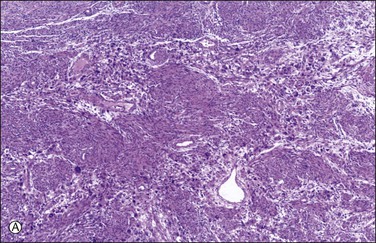
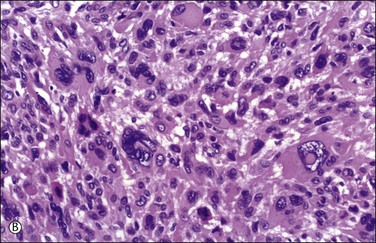
Figure 19.13 Bizarre leiomyoma. (A) ‘Zebra-like’ pattern. Zones of bizarre smooth muscle cells alternate with bands of uninvolved ordinary leiomyoma. (B) Cluster of bizarre smooth muscle cells with hyperchromatic and hyperlobated nuclei containing prominent eosinophilic cytoplasmic pseudoinclusions. No mitotic figures are present.
Leiomyomas with bizarre nuclei are distinguished from leiomyosarcomas by an absence of tumor cell necrosis and mitotic counts of <10 mitotic figures per 10 HPFs. A mitotic index higher than 10 mitotic figures per 10 HPF in an atypical uterine smooth muscle tumor is diagnostic of malignancy. However, otherwise clear-cut leiomyosarcomas may contain areas indistinguishable from bizarre leiomyomas. In such cases, the finding of atypical mitotic figures and tumor cell necrosis helps in establishing the correct diagnosis (Figure 19.15).
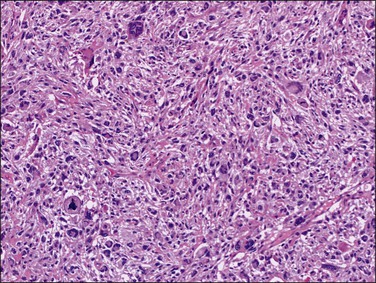
Figure 19.15 Leiomyosarcoma. Focally, this pleomorphic leiomyosarcoma may resemble a bizarre leiomyoma, but the presence of abnormal mitoses is indicative of malignancy.
Unfortunately, in the 2003 World Health Organization (WHO) classification of mesenchymal neoplasms of the uterus, ‘leiomyoma with bizarre nuclei’ was renamed as ‘atypical leiomyoma.’ Furthermore, uterine smooth muscle tumors with borderline atypia and mitotic activity were designated as STUMP.71 This change of terminology has created controversy and is not universally accepted. Whereas the bizarre nuclear changes of leiomyomas are qualitative and readily identified microscopically, the term ‘atypical’ leiomyoma best describes a smooth muscle tumor exhibiting nuclear atypicality and mitotic activity quantitatively insufficient for the diagnosis of leiomyosarcoma, i.e., STUMP. In fact, we think the term ‘atypical smooth muscle tumor’ is preferable to STUMP because it avoids the word ‘malignant,’ does not imply uncertainty, and does not cause unnecessary concern to patients.
Immunohistochemical and Cytogenetic Features
Most bizarre leiomyomas are immunoreactive for p16 (86.5%) and approximately 60% immunoreact for p53. Nearly half of these tumors show over 10% of cells positive for Ki-67. Thus, because of significant overlapping staining patterns between leiomyosarcoma and bizarre leiomyoma, immunoreactions for p16, p53, and Ki-67 have a limited role in distinguishing these two tumors.72
Nearly half of bizarre leiomyomas show loss of heterozygosity for loci on the short arm of chromosome 1, suggesting that they might harbor a tumor suppressor gene for atypical smooth muscle tumors on 1p.51 Interestingly, the expression profiles of bizarre leiomyomas with 1p more closely resemble that of leiomyosarcomas than the profiles of myometrium and leiomyomas of the usual histologic type.51
Epithelioid Leiomyoma
Epithelioid leiomyomas are composed of rounded or polygonal cells rather than the usual spindle-shaped cells of ordinary leiomyomas. They are also known as leiomyoblastomas, clear cell leiomyomas, or plexiform leiomyomas.73–75
Grossly, these tumors may resemble typical leiomyomas or appear fleshy due to their high cellularity. They may be softer and more yellow than the ordinary non-epithelioid type leiomyoma (Figure 19.16). The average diameter is 6–7 cm. The tumor cells are usually arranged in nests or cords, and show abundant cytoplasm, rounded nuclei with finely stippled chromatin, and a single nucleolus (Figure 19.17). Leiomyoblastomas contain rounded cells with ample eosinophilic cytoplasm (Figure 19.18). The cells in clear cell leiomyomas are polygonal and have abundant clear cytoplasm, which may contain glycogen (Figure 19.19). Sometimes the nucleus is eccentric, resulting in a signet-ring appearance. Plexiform leiomyomas show cords or nests of rounded cells with scanty to moderate amounts of cytoplasm. These tumors may appear as multiple, microscopic foci, which have been referred to as ‘plexiform tumorlets.’ The histologic phenotype of plexiform tumors may be more a result of abundant elaboration of extracellular matrix material than of epithelioid differentiation. A transition to more typical spindled smooth muscle cells is frequently seen within an epithelioid leiomyoma and mixtures of the various patterns are common.
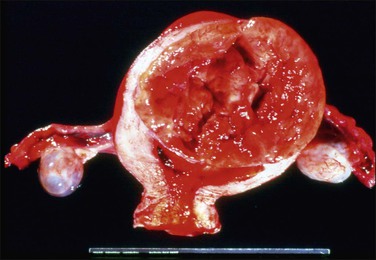
Figure 19.16 Epithelioid leiomyoma. Compared to the ordinary leiomyoma, the tumor appears fleshy and shows yellow and hemorrhagic areas.
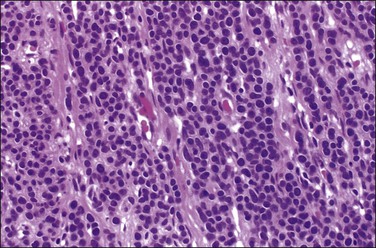
Figure 19.17 Epithelioid leiomyoma. The tumor cells are arranged in nests or cords and show abundant cytoplasm, rounded nuclei with finely stippled chromatin, and a single nucleolus.
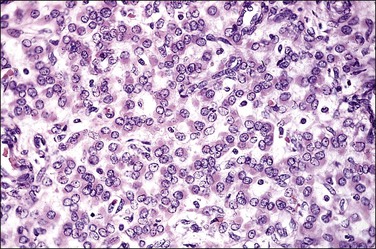
Figure 19.18 Epithelioid leiomyoma, leiomyoblastoma type. The tumor is composed of uniform, round cells with eosinophilic cytoplasm.
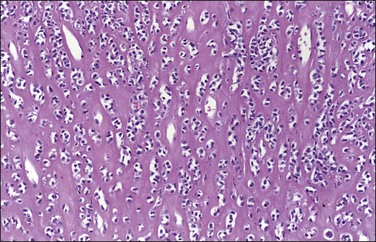
Figure 19.19 Epithelioid leiomyoma, clear cell type. Polygonal cells with abundant clear cytoplasm in a hyalinized stromal background.
The smooth muscle nature of these variants of epithelioid leiomyoma has been confirmed by immunohistochemistry and electron microscopy.73 Ultrastructural studies have revealed features of smooth muscle differentiation such as parallel cytoplasmic filaments, dense bodies, and basal lamina.
Because of the rarity of epithelioid smooth muscle tumors, criteria predictive of their malignant behavior are less well established than those for spindle-cell smooth muscle tumors.72 In an old study of 26 cases, small size, expansile margin, presence of clear cytoplasm, extensive hyalinization, and lack of tumor cell necrosis were parameters associated with a favorable prognosis; in contrast, larger tumors (>6 cm) that exhibited >5 mitotic figures per 10 HPF were designated as epithelioid leiomyosarcomas based on their metastatic potential.75 Intermediate tumors with moderate to severe atypia, without necrosis, and <5 mitotic figures per 10 HPF should be classified as atypical or borderline leiomyomas (so-called STUMPs) and patients should be followed.76 In summary, extensive epithelioid differentiation of a uterine smooth muscle tumor is a disturbing finding because the absence of nuclear atypia and tumor cell necrosis does not warrant a favorable behavior when the tumor contains 5 mitotic figures per 10 HPF.77 However, the risk of recurrence is probably low. Epithelioid leiomyomas should be distinguished from carcinomas, especially those composed of eosinophilic or clear cells, PEComas, placental site trophoblastic tumors (PSTTs) or epithelioid trophoblastic tumors (ETTs), and low-grade endometrial stromal sarcomas. Desmin immunoreactivity; absence of the characteristic features of PEComa, PSTT, and ETT; and lack of vascular space invasion typically found in low-grade endometrial stromal sarcoma facilitate the correct diagnosis.
Myxoid Leiomyoma
Myxoid leiomyoma is a benign smooth muscle tumor with extensive myxoid change that may occur during pregnancy.78,79 Grossly, it resembles an extrauterine myxoma. Microscopically, it shows well-defined borders and contains abundant, acellular, pale-staining material rich in acid mucins that stain with Alcian blue or colloidal iron. The tumor cells may be elongated or stellate in shape and are widely separated by the extracellular material. Cytologic features are bland and mitotic figures are rare. In curettage specimens, distinction between myxoid leiomyoma and myxoid leiomyosarcoma may be difficult. Non-myxoid portions of the leiomyoma may be erroneously interpreted as evidence of myometrial invasion. In an unpublished study,78 a mitotic index of <2 mitotic figures per 10 HPF in the absence of tumor cell necrosis or severe cytologic atypia favored the diagnosis of myxoid leiomyoma. However, large myxoid smooth muscle tumors and those with an infiltrating margin, exhibiting moderate to severe nuclear atypia, with or without necrosis and any mitotic index, should be regarded as myxoid leiomyosarcomas.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree