Ovarian Germ Cell Tumors
General Features
Germ cell tumors arise from primordial germ cells and account for approximately 30% of all ovarian tumors. Over 95% of them are benign dermoid cysts (mature cystic teratomas) and the remaining 5% are malignant. Malignant germ cell tumors (MGCTs) represent approximately 3% of all ovarian cancers in Western countries and about 20% in Asian and African populations, where surface epithelial cancers are much less common.1
Germ cell tumors replicate in a distorted form various stages of normal embryogenesis and, like the embryo, are capable of developing complex and highly differentiated tissues.2 The malignant potential of ovarian germ cell tumors is inversely related to their degree of differentiation. Some traditional views on the histogenesis of germ cell tumors have been recently challenged. Although germ cell tumors of the ovary are morphologically similar to testicular germ cell tumors, they may not necessarily have an identical origin.3 Testicular germ cell tumors originate from primitive germ cells with a malignant character, whereas ovarian germ cell tumors have a parthenogenetic origin from postmeiotic or meiotic cells.4 For this reason, embryonal carcinoma (EC) occurs more frequently in the testis than the ovary. Nevertheless, an unknown percentage of MGCTs developing in phenotypic females may, in fact, represent testicular germ cell tumors (seminomas, ECs, mixed germ cell tumors), as they may have originated from the malignant germ cell component of gonadoblastomas present in dysgenetic gonads of patients with an unrecognized Y chromosome-containing genotype.5 Rarely, germ cell tumors may arise from pre-existing somatic neoplasms of the female genital tract.6–9 In these cases, the teratoid tumors derive most likely from a pluripotent stem cell population of somatic neoplasms. Recent evidence suggests that germinomas may not be end-stage tumors, as traditionally believed, but some of them could be precursors to germ cell neoplasms capable of further differentiation.10–15 The occasional morphologic overlap between the primitive or immature forms of germ cell tumors, such as germinoma, EC, and yolk sac tumor (YST), supports the view that these tumors constitute a closely related group of neoplasms capable of embryonic or extraembryonic differentiation.10,16 The new concepts of tumor histogenesis have been represented in a tridimensional tetrahedron model of inter-relationships between the different components (Figure 29.1). Nevertheless, the most popular histogenetic scheme17 (shown in Figure 29.2) forms the basis for the current World Health Organization (WHO) classification of ovarian germ cell tumors (Table 29.1).18
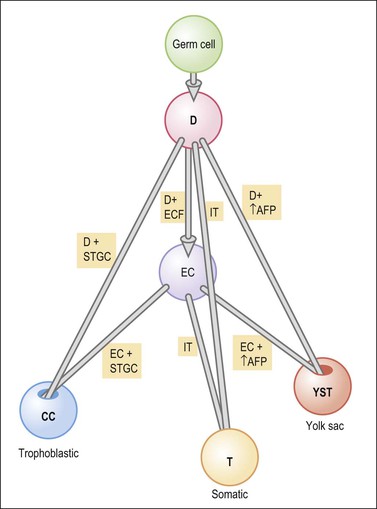
Figure 29.1 Tetrahedron model of germ cell histogenesis. D, dysgerminoma; D+ECF, dysgerminoma with early carcinomatous features; EC, embryonal carcinoma; D+STGC, dysgerminoma with syncytiotrophoblastic giant cells; D+↑AFP, dysgerminoma with elevated α-fetoprotein; IT, immature teratoma; EC+STGC, embryonal carcinoma with syncytiotrophoblastic giant cells; EC+↑AFP, embryonal carcinoma with elevated α-fetoprotein; CC, choriocarcinoma; T, mature teratoma; YST, yolk sac tumor.11 (Modified with permission of Taylor & Francis.)
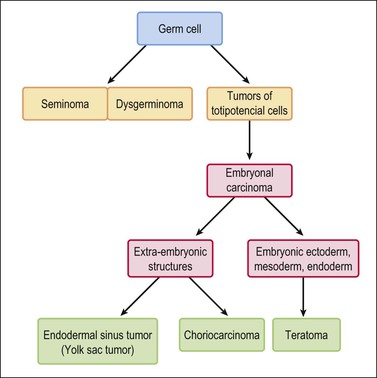
Figure 29.2 Germ cell tumors. Traditional histogenetic scheme.17 (Data from Teilum G. Special tumors of the ovary and testis. Comparative pathology and histological identification. JB Lippincott, Philadelphia, 1976: p. 68)
MGCTs generally occur in younger women (75% in women under 30 years) and account for two-thirds of ovarian cancers in the first two decades.1,19 The tumors are usually large (median size is 16 cm). Bilateral tumors are rare except dysgerminomas (10–20% bilaterality). Abdominal enlargement and pelvic pain are the most common presenting symptoms. Teenagers who present with abdominal masses and who have never menstruated should be evaluated for the possibility of a gonadoblastoma that has undergone malignant progression. Preoperative karyotyping can be helpful to identify underlying chromosomal abnormalities. Human chorionic gonadotropin (hCG) and α-fetoprotein (AFP) levels are useful markers in the diagnosis and in monitoring the postoperative course of these patients. Moreover, recent stem cell research has provided several highly diagnostic pluripotency markers, including transcription factors and cytoplasmic/membranous proteins that are sequentially expressed in MGCTs according to their differentiation stage.20,21
MGCTs are now frequently cured by fertility-sparing surgery and combination chemotherapy including bleomycin, etoposide, and cisplatin.22 For patients with advanced stage disease, maximum cytoreductive surgery appears to be beneficial. About 60–75% of women have stage I disease and 25–30% have stage III disease. For patients with early stage disease, cure rates approach 100%. For those with advanced stage disease, cure rates are reportedly at least 75%.22
The relative frequencies of the various types of MGCTs are shown in Table 29.2. Most MGCTs occur in a pure form, but approximately 15% of cases contain two or more types. The prognosis of patients with a mixed MGCT usually reflects that of its most malignant component. Therefore, it is important to sample these tumors extensively, particularly areas with different gross appearance. One section per every centimeter in tumor diameter is recommended. Most MGCTs are composed of primitive tissues (i.e., dysgerminoma, YST); less frequently, malignant neoplasms of the adult type arise in dermoid cysts, usually in older patients.
Table 29.2
Approximate Frequency of MGCTs of the Ovary
| • Dysgerminoma | 33% |
| • YST | 10% |
| • EC | — |
| • Polyembryoma | — |
| • Choriocarcinoma | <1% |
| • Teratoma | |
| • Immature | 36% |
| • Malignancy in dermoid cyst | 5% |
| • Monodermal | — |
| • Mixed | 15% |
Dysgerminoma
Clinical Features
Dysgerminoma is one of the most common MGCTs of the ovary, but it accounts for only 1–2% of all ovarian cancers. They occur almost exclusively in children and young women. The average patient age is 22 years.23 Most patients present with a rapidly growing abdominal mass that often causes lower abdominal pain or pressure and may simulate pregnancy. Premenarchal patients with a pelvic mass should have their karyotype determined, as approximately 5% of dysgerminomas arise from gonadoblastomas in phenotypic females with abnormal gonads. These patients have pure gonadal dysgenesis (46,XY, bilateral streak gonads) or mixed gonadal dysgenesis (45,X/46,XY, unilateral streak gonad, contralateral testis). The estimated risk of malignancy is 28% by the age of 20 years for the former patients and 19% risk by the same age for the latter. Therefore, prophylactic removal of the gonads is recommended in both groups of patients at an early age.1 Almost all patients with dysgerminoma have elevated serum levels of lactic dehydrogenase (1 and 2 fractions) at presentation.24 In rare cases (3–5%), dysgerminomas may produce hCG and these patients may present with endocrine symptoms that are characteristically estrogenic (menstrual irregularities, isosexual pseudoprecocity, pseudopregnancy), but rarely androgenic.25 Serum levels of CA125, placental-like alkaline phosphatase (PLAP), and neuron-specific enolase (NSE) have been elevated in some cases. Paraneoplastic hypercalcemia has been described in some patients.26 One tumor occurred in a patient with a germline BRCA1 mutation.27 About two-thirds of dysgerminomas are stage IA at diagnosis; higher stage tumors involve the contralateral ovary (20%), pelvic and para-aortic lymph nodes, and/or the pelvic and abdominal peritoneum.
Macroscopic Features
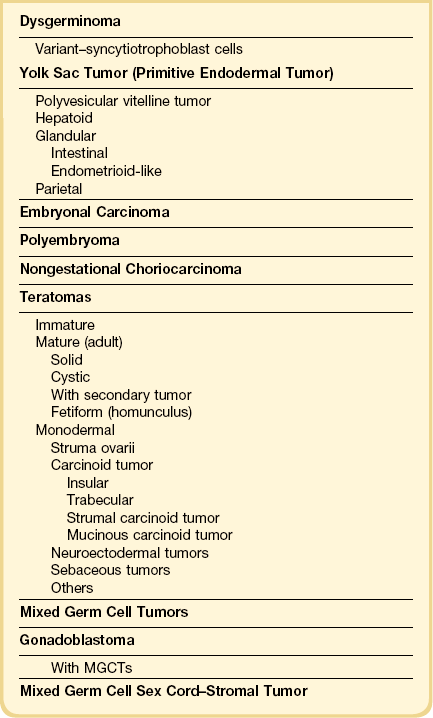
Dysgerminomas are characteristically solid, and are well-encapsulated tumors with an average diameter of 15 cm. On section, they are lobulated, soft, and fleshy, and may appear gray-white or light tan (Figure 29.3). Areas of coagulative necrosis and hemorrhage typically associated with cystic change may be seen. Such areas should be sampled to rule out the presence of other types of MGCT. The presence of calcification suggests an underlying gonadoblastoma. Dysgerminoma is the only MGCT that has a significant rate of bilaterality. Gross involvement of the contralateral ovary is seen in 10% of cases, and in another 10% microscopic foci of tumor are found.1
Microscopic Features
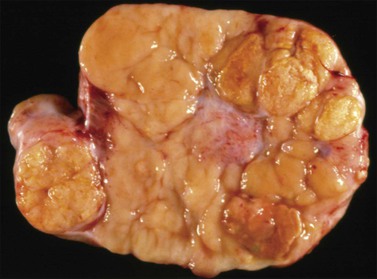
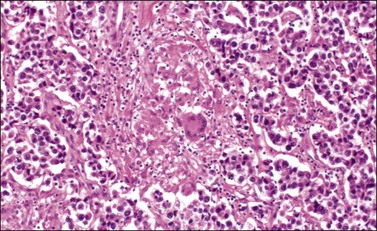
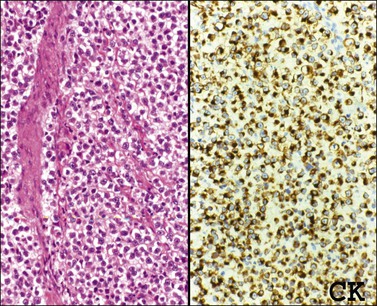
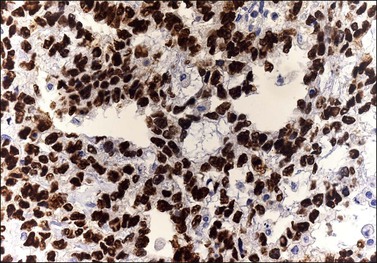
The microscopic appearance of dysgerminoma is identical to that of testicular seminoma and extragonadal germinoma. It is composed of a monotonous population of rounded cells resembling primordial germ cells in a predominantly diffuse or insular arrangement. The cells may also grow as trabeculae, cords, or small nests (Figure 29.4). Lack of intercellular cohesion may result in the formation of pseudoglandular spaces. The tumor cells are polygonal, with discrete cell membranes and abundant eosinophilic to clear, glycogen-rich, cytoplasm. The nuclei are large, central, and rounded, and they contain one or a few prominent nucleoli. Mitotic figures are often numerous. Aggregates of tumor cells are usually separated by thin fibrous septa almost always infiltrated by T-lymphocytes.28 In some tumors, lymphocytes are abundant and may form follicles with germinal centers. In approximately 20% of the cases, epithelioid sarcoid-like granulomas with multinucleated giant cells are present (Figure 29.5).1 About 5% of dysgerminomas contain syncytiotrophoblastic giant cells (SGCs) in the absence of any other non-germinomatous differentiation.25 Such tumors, which have the same prognosis as dysgerminomas in which SGCs are absent, should be sampled extensively to rule out foci of choriocarcinoma or EC. The SGCs have a perivascular location, are immunoreactive for human chorionic gonadotropin (hCG)(Figure 29.6), and are often associated with stromal luteinization. The luteinized stromal cells, which may be admixed with the tumor cells or located at the periphery of the neoplasm,29 are probably responsible for the estrogenic or androgenic symptoms that are found in some patients.
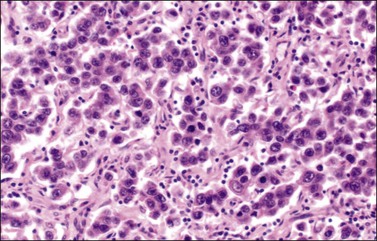
Figure 29.4 Dysgerminoma. The tumor cells are distributed in nests separated by delicate fibrous septa. The stroma contains numerous lymphocytes.
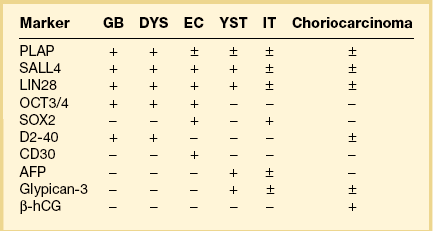
Immunohistochemistry
Dysgerminoma cells show cytoplasmic and membranous immunoreaction for vimentin, PLAP, CD117 (c-kit), and D2-40 (podoplanin); the membranous reaction is characteristic of dysgerminoma.30,31 There is nuclear immunoreaction for the stem cell/primitive germ cell nuclear transcription factors OCT3/4, NANOG, and SALL431–33 (Table 29.3). OCT3/4 is expressed very early during embryogenesis and has an essential role in blastocyst differentiation. However, when the female germ cells enter meiosis the expression of OCT3/4 is downregulated. OCT3/4 regularly shows positivity in dysgerminoma (Figure 29.7) but can also be expressed in EC and in some immature neural elements of ovarian teratoma.
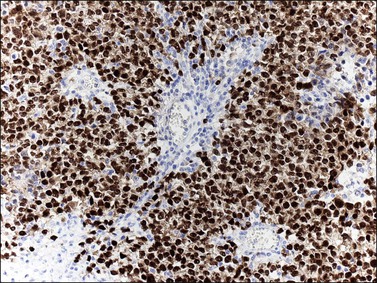
Figure 29.7 Dysgerminoma. OCT3/4-positive nuclear immunoreaction recognizes germ cells in poorly fixed specimen. (Courtesy of Dr. Francisco Nogales.)
SALL4 can also show positivity in EC, YST, and primitive areas of immature teratoma (IT).33 Dysgerminomas may exhibit limited cytoplasmic dot- or rim-like staining for cytokeratin (Figure 29.8) but epithelial membrane antigen (EMA) is negative. The SGCs that are found in a small percentage of dysgerminomas exhibit positive cytoplasmic staining for hCG (Figure 29.6).
Somatic Genetics
Dysgerminomas, like seminomas, show by comparative genomic hybridization frequent (75%) chromosome 12p gain, usually in the form of an isochromosome 12p.34 The c-kit gene encodes a tyrosine kinase receptor (KIT), which is required in normal spermatogenesis and is expressed in seminomas and dysgerminomas. c-kit point mutations localized to exon 17, codon 816 (Asp>Val), involving the phosphotransferase domain, have been identified in 25–50% of ovarian dysgerminomas.35–37 The mutations are in exon 17, not in the exon 11 location, which confers susceptibility to imatinib therapy.
Differential Diagnosis
Dysgerminoma should be distinguished from the solid type of YST, EC, clear cell carcinoma, and large cell lymphoma. The solid YST is almost always associated with other more typical patterns that are not seen in dysgerminomas. YSTs exhibit greater nuclear variation, contain hyaline bodies, immunoreact for AFP and glypican-3, and lack the lymphocytic infiltrate of dysgerminomas. Moreover, in contrast to dysgerminoma, YSTs fail to react for D2-40 and OCT3/4. The extremely rare EC shows larger cells with more ample nuclei that are more hyperchromatic and irregular than those of dysgerminoma. ECs are CD30 and cytokeratin positive, almost always contain SGCs, and lack the stromal infiltrate of lymphocytes. The distinction of dysgerminoma from clear cell carcinoma is discussed in Chapter 27. Large cell lymphomas may resemble dysgerminomas both grossly and microscopically. Lymphomas, however, are bilateral in approximately one-half of the cases, and simultaneous involvement of the ipsilateral tube occurs in 25% of them38 (see Chapter 30). Careful attention to the nuclear features and immunostains for PLAP, D2-40, SALL4, and lymphoid markers facilitate the correct diagnosis. Poorly fixed dysgerminomas may occasionally be misdiagnosed as poorly differentiated carcinomas, as the cellular features of the former tumors are lost.
Treatment and Prognosis
The treatment of patients with dysgerminoma is primarily surgical, including the resection of the ovarian tumor and complete surgical staging. Given that the tumor mainly affects young females, for whom preservation of fertility is important, unilateral adnexectomy with frequent patient follow-up (every 2–3 months for the first 2–3 years) is the treatment of choice. This conservative approach is currently used in young patients even in the presence of metastasis.39 Therefore, biopsy of an apparently normal contralateral ovary is not recommended as it increases the risk of infertility. Dysgerminoma is highly sensitive to chemotherapy, which is usually reserved for the treatment of recurrent disease.23,40 Although these tumors are equally sensitive to radiotherapy, its use has been discontinued in an effort to preserve patient fertility, and cisplatin-based chemotherapy is currently preferred.22 In young patients whose karyotype analysis reveals a Y chromosome, both ovaries should be removed.1 When the patient’s fertility is not a factor, hysterectomy with bilateral salpingo-oophorectomy is recommended; chemotherapy is subsequently administered in the case of higher stage tumors.22 Postoperative work-up includes the serum markers AFP and hCG, chest X-ray, and abdominopelvic CT or MRI. Recurrence in the retained contralateral ovary can occur in 5–15% of cases over the next 2 years.1 About 75% of the recurrences occur within the first postoperative year, and the most common sites are the peritoneal cavity and the retroperitoneal lymph nodes.1
Patients with dysgerminoma have an excellent prognosis. The 5 year survival rate is greater than 95% for patients with stage IA tumor, and 85% for those with advanced stage or recurrent tumor.22 Features associated with recurrence include a tumor diameter greater than 10 cm, and age younger than 20 years.41 Although the designation ‘anaplastic dysgerminoma’ has been applied to tumors with numerous mitoses, there is no evidence that such tumors are associated with a worse prognosis, and therefore this term is not recommended. Most dysgerminomas are nondiploid and ploidy is not a useful prognostic factor.42
Yolk Sac Tumor (Primitive Endodermal Tumor)
Clinical Features
Yolk sac tumors (YSTs) (also referred to as primitive endodermal tumors) account for approximately 10% of MGCTs and are almost as common as dysgerminoma in patients under the age of 20 years.1 They occur at a median age of 18 years and are rare over the age of 40 years.1,43–45 Patients most frequently present with abdominal pain and a large, rapidly growing, pelvic mass. Rupture or torsion of the tumor occurs in about 10% of patients. YSTs consistently produce AFP, which can be demonstrated in the patient’s serum, usually at a level greater than 1000 ng/ml. This substance, which is normally produced in the yolk sac of the developing embryo, may serve as a tumor marker in monitoring the patient during and after therapy. Lower serum levels of AFP may be encountered in other tumors that also occur in young females such as IT and Sertoli–Leydig cell tumors (SLCTs). CA125 and carcinoembryonic antigen (CEA) are also elevated in 100% and 10% of YSTs, respectively. Rare examples of YST have occurred in older patients in association with mucinous or endometrioid tumors; in such cases, a somatic cell rather than a germ cell origin is almost certain.6,8,9
Yolk sac tumor is a highly malignant neoplasm. At laparotomy, evidence of extraovarian spread has been reported in 30–70% of cases.44,45 In an older study,46 subclinical metastases were present in 84% of cases regarded as ‘stage I’ tumors, which most likely reflects incomplete staging. When the tumor spreads beyond the ovary, it involves the omentum and peritoneum, the para-aortic lymph nodes, and the liver.
Macroscopic Features
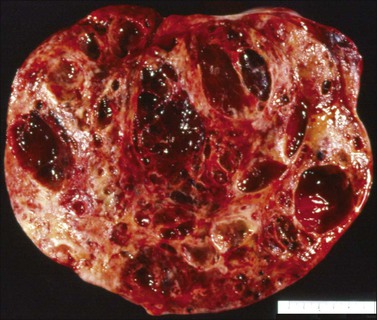
Yolk sac tumors are large, well-encapsulated masses, with an average diameter of 15 cm. The external surface is usually smooth and glistening. The sectioned surface is characteristically solid and cystic and shows soft, gray to yellow tissue with extensive areas of hemorrhage and necrosis (Figure 29.9). When a polyvesicular vitelline component is present, the tumor shows a microcystic appearance.1 Although YSTs are bilateral in less than 5% of patients, the contralateral ovary may contain a dermoid cyst in about 10% of cases.1
Microscopic Features
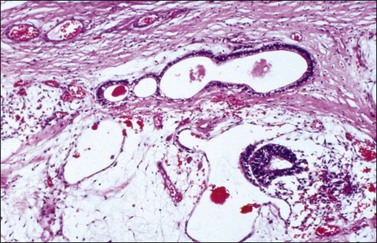
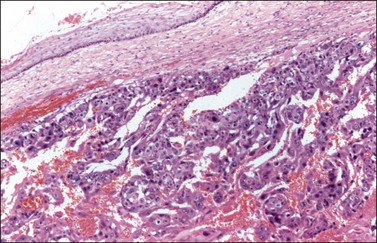
Although YSTs often exhibit a wide variety of microscopic patterns, most tumors have a reticular architecture reflecting extraembryonic differentiation.46,47 They are composed of a network of irregular spaces lined by primitive epithelial cells with glycogen-rich, clear cytoplasm, and large, hyperchromatic nuclei with prominent nucleoli; mitotic figures are numerous (Figure 29.10). The most characteristic feature is the presence of isolated papillary projections with a central blood vessel and peripheral sleeve of embryonic epithelial cells (Schiller–Duval bodies; Figure 29.11). Cross sections of this structure once were erroneously compared with immature glomeruli (Schiller’s mesonephroma).48 In fact, they closely resemble invaginations of yolk sac endoderm, as seen best in the rat placenta, forming the endodermal sinuses of Duval.46 Nevertheless, Schiller–Duval bodies are present in only 20% of cases.47 The term ‘endodermal sinus tumor’ is misleading, since the endodermal sinus is neither a structure present in human embryogenesis nor a constant feature of these neoplasms. Accordingly, the designation primitive endodermal tumor has recently been proposed.49 Another distinctive feature is the presence of brightly eosinophilic, periodic acid–Schiff (PAS)-positive, diastase-resistant hyaline globules (Figure 29.10). Occasionally, multiple small vesicles with eccentric constrictions resembling the yolk sac vesicle of the normal embryo are present, and the tumor is designated a polyvesicular vitelline tumor (Figure 29.12).46,50,51 This pattern recapitulates the subdivision of the primary yolk sac vesicle into a large component lined by flat cells (vestigial primary yolk sac) and a smaller component lined by taller epithelium that simulates the forerunner of the primitive gut and its appendages (secondary yolk sac vesicle) in normal embryogenesis (Figure 29.13). Like the normal yolk sac vesicle, the neoplastic yolk sac may give rise to tumors of embryonal type. These tumors recapitulate primitive gut (glandular YST) and primitive liver (hepatoid YST).52–56
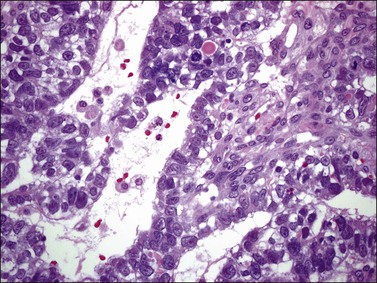
Figure 29.10 Yolk sac tumor. Reticular pattern. Irregular spaces lined by primitive epithelial cells with glycogen-rich, clear cytoplasm, and large, hyperchromatic nuclei with prominent nucleoli. A hyaline body is present (upper center). Mitotic figures are seen.
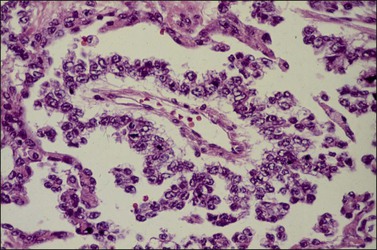
Figure 29.11 Yolk sac tumor. Schiller–Duval (glomeruloid) body. Central blood vessel and peripheral sleeve of embryonic epithelial cells.
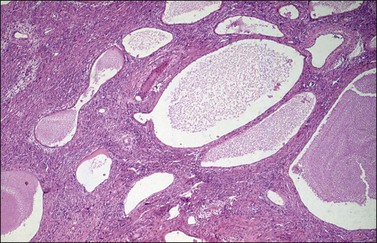
Figure 29.12 Yolk sac tumor, polyvesicular vitelline variant. The vesicles are lined by flattened epithelial cells and some of them exhibit eccentric constrictions.
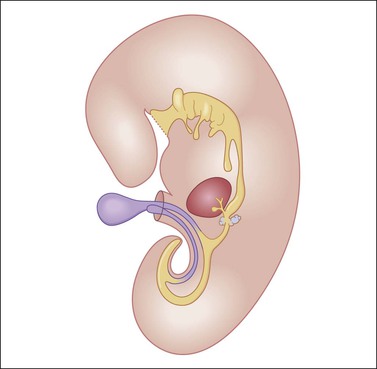
Figure 29.13 Diagram of a median longitudinal section through a 10 mm human embryo (about 6 weeks old). The primitive yolk sac (blue) is in continuity with the midgut. The rudiment of the liver and biliary tract appears attached to the ventral wall of the foregut. (Modified from Prat J, Pathology of the ovary. Saunders, Philadelphia, 2004. With permission.)
Endodermal-type glands may be found in approximately 50% of YSTs, often admixed with reticular and polyvesicular vitelline components.1 In some tumors, a glandular pattern predominates, and may appear as rounded cribriform aggregates of primitive epithelial cells (‘intestinal’ variant; Figure 29.14),52 or glands of ‘endometrioid’ type, which can be mistaken for typical or secretory endometrioid adenocarcinoma (endometrioid-like variant; Figure 29.15);53 foci of carcinoid tumor have been described in one of the latter tumors.57 Occasionally, the mature intestinal component may give rise to a mucinous carcinoid.58
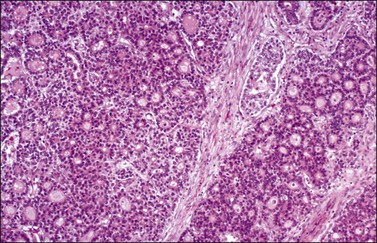
Figure 29.14 Yolk sac tumor, glandular variant, intestinal type. The tumor shows a cribriform pattern.
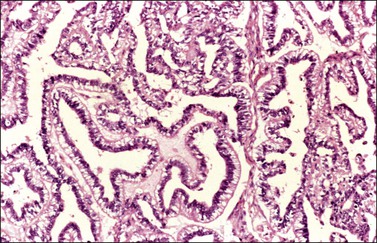
Figure 29.15 Yolk sac tumor, endometrioid-like glandular variant. The tumor glands resemble those of the secretory endometrium.
Although small foci of hepatoid differentiation are found in 16–48% of YSTs,55,56 neoplasms with a predominant hepatoid component are infrequent.54,59 These tumors are characterized by compact masses of large polyhedral cells with abundant eosinophilic cytoplasm, and round central nuclei with prominent single nucleoli, separated by thin fibrous bands, resembling hepatocellular carcinoma (Figure 29.16). Hyaline bodies are usually numerous. In some cases, glandular spaces containing mucin are present. Ultrastructural examination of hepatoid YSTs reveals features similar to hepatocellular carcinoma.54 A ‘parietal’ differentiation characterized by small extracellular accumulations of basement membrane material has been described in over 90% of YSTs.55 Rarely, YSTs may contain SGCs.
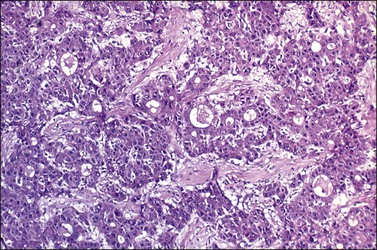
Figure 29.16 Yolk sac tumor, hepatoid variant. The tumor is composed of compact masses and nests of large polyhedral cells with abundant eosinophilic cytoplasm separated by a fibrous stroma. Note the striking resemblance to a hepatocellular carcinoma.
The loose stromal component of YST recapitulates the extraembryonic mesenchyme (magma reticulare),46 and may be the site of origin of the sarcomas that develop in some patients after chemotherapy.60,61
Immunohistochemistry
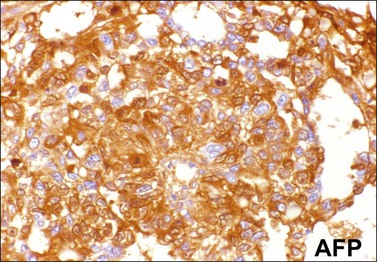
Similar to the primitive gut (secondary yolk sac) and its appendages, YSTs are immunoreactive for AFP (Figure 29.17), glypican-3 (Figure 29.18), SALL4 (Figure 29.19), villin, CDX2. and hepatocyte paraffin antigen 1.33 AFP is expressed often focally either as granular cytoplasmic deposits or delineating intracellular canaliculi. Glypican-3 immunoreaction is usually stronger than that of AFP;62 however, it is not as specific since it is expressed by other germ cell tumors and clear cell carcinoma.63 Nuclear transcription factor SALL4 is regularly expressed in both epithelium and mesenchyme32 and there is cytoplasmic reactivity of RNA-binding protein LIN28.64 Villin is also constantly expressed in membranes and cytoplasms of epithelial cells. The different endodermal elements may be immunoreactive for their corresponding tissue markers, i.e., hepatic components for hepatocyte paraffin antigen 1,65 intestinal elements for CDX2,49 and foregut-derived epithelia for thyroid transcription factor 1.33 However, YSTs are negative for OCT4, SOX2, D2-40, and CD30 (Table 29.3). Combined expression of AFP, glypican-3, villin, SALL4, or LIN28 makes an ideal panel for confirming the diagnosis.49
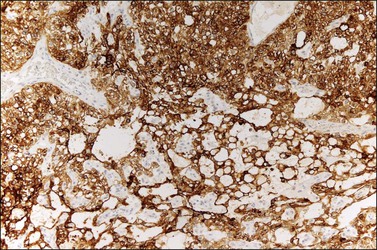
Figure 29.18 Yolk sac tumor. Glypican-3 immunoreaction delineates a clean microcystic pattern. (Courtesy of Dr. Francisco Nogales.)
Differential Diagnosis
Because of their various microscopic patterns, YSTs can be confused with many other ovarian tumors, both primary and metastatic. The most common problems in differential diagnosis include clear cell carcinoma and endometrioid adenocarcinoma (see Chapter 27), the retiform variant of SLCT (see Chapter 28), and hepatoid carcinoma (see Chapter 27). YSTs should also be distinguished from other germ cell tumors such as dysgerminoma and the very rare EC. These differential diagnoses are discussed under those headings.
Treatment and Prognosis
The treatment of YST includes surgical exploration, unilateral salpingo-oophorectomy, and frozen section for diagnosis.22,44 Although gross metastases should be removed, a thorough surgical staging is not necessary because all patients need chemotherapy.44 Prior to combination chemotherapy, the prognosis for patients with YSTs was dismal despite apparently adequate surgery. In one large study of patients treated prior to 1975 the 3 year survival was only 13%.47 The development of the vincristine, dactinomycin, and cyclophosphamide (VAC) combination chemotherapy dramatically changed the outlook for patients with YSTs. Currently, cisplatin-based chemotherapy provides still better results. Survival rates have approached 100% for patients with stage I tumors, and 75% for patients with higher stage tumors.22 Serum AFP can be used to monitor the response to treatment and to detect tumor recurrence. Adverse prognostic factors include tumor stage II or higher, gross residual tumor after surgery, and ascitic fluid of more than 100 ml.45 The good long-term outcome in two polyvesicular-vitelline tumor (PVVT) cases supports prior evidence that this form of YST may be more indolent than the conventional forms of the tumor.51
Embryonal Carcinoma
Embryonal carcinoma (EC) is a very rare ovarian tumor that is morphologically identical to EC of the testis. For many years, it was confused with YST, which it resembles.66 From a histogenetic viewpoint, EC has been considered a pluripotent stem cell tumor capable of differentiating along different pathways.67 The patients are young (median age, 12 years), present with an abdominal mass, and consistently have a positive pregnancy test as a result of hCG production by the tumor.66 Premenarchal patients have endocrine manifestations in about half of the cases; usually, they include isosexual pseudoprecocity, irregular bleeding, amenorrhea, or hirsutism. Laparotomy reveals peritoneal spread in 40% of cases.66 Grossly, ECs are large (17 cm), unilateral tumors that exhibit solid and variegated cut surfaces. Foci of hemorrhage and necrosis are common. Histologically, ovarian EC resembles testicular Embryonal carcinoma; it is composed of large primitive cells distributed in solid masses, often exhibiting central necrosis, gland-like spaces, and papillae. The nuclei are round and vesicular. Mitotic figures, including atypical forms, are numerous. Hyaline globules are usually present. SGCs immunoreactive for hCG are almost always present (Figure 29.20).
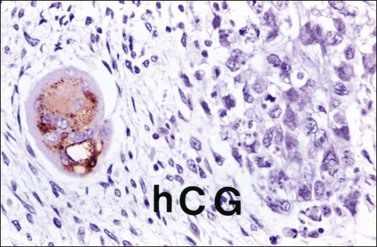
Figure 29.20 Embryonal carcinoma. A syncytiotrophoblastic giant cell shows positive immunostaining for hCG.
Embryonal carcinoma is most often found in the ovary as a component of a mixed germ cell tumor. The tumor cells are immunoreactive for cytokeratin, PLAP, CD30, NSE, AFP, and hCG. The last two substances can be used as tumor markers. Nuclear transcription factors OCT3/4 and SALL4 are positive and SOX2 is variably positive.31,32,68 Immunoreaction for EMA is negative. Most ECs contain an isochromosome, i12p. EC should be distinguished from other MGCTs (dysgerminoma, YST), juvenile granulosa cell tumor (JGCT), and undifferentiated carcinomas in the surface-epithelial category. In contrast to EC, the last two tumors rarely produce AFP and usually lack SGCs. Unilateral salpingo-oophorectomy is the recommended surgical procedure. Although these tumors are highly malignant, cisplatin-based combination chemotherapy has resulted in some long-term survivors.
Polyembryoma
Rare cases of MGCT contain large numbers of embryoid bodies in various stages of development, typically distributed in a primitive mesenchymal stroma.69–72 Only 15 ovarian polyembryomas have been reported in the English literature, most of them found as components of mixed germ cell tumors in children or young women.72 Serum levels of AFP and hCG may be elevated. Grossly, polyembryomas are unilateral large tumors with a microcystic cut surface. Microscopically, the embryoid bodies resemble perfect or imperfect early embryos and contain germ discs, yolk sacs, amniotic cavities, chorionic elements, and extraembryonic mesenchyme in an edematous stroma (Figure 29.21). Well-differentiated enteric glands and mature or immature hepatic tissue, which may secrete bile, are found in some cases. The yolk sac components of the embryoid bodies and the nests of liver cells are immunoreactive for AFP. The behavior of polyembryomas is similar to that of other MGCTs. Conservative surgery and combination chemotherapy is the recommended treatment.
Nongestational Choriocarcinoma
Pure nongestational choriocarcinoma of the ovary is an exceedingly rare and highly malignant tumor that develops before puberty. It accounts for less than 1% of MGCTs.1 More frequently, choriocarcinoma is seen as a component of a mixed MGCT73 (only 45 pure cases have been reported).74 Clinically, these patients present with abdominal enlargement and pain and, occasionally, there is hemoperitoneum simulating a tubal pregnancy.75 The pregnancy test is positive and the elevated serum level of hCG may lead to isosexual pseudoprecocity in children or menstrual abnormalities in older patients.75 Serum β-hCG levels range from hundreds to more than 2,000,000 mIU/ml.74 Grossly, choriocarcinomas are large (4–25 cm), hemorrhagic, and friable tumors. Microscopically, much of the tumor is hemorrhagic and necrotic. The viable areas show cytotrophoblastic and syncytiotrophoblastic cells arranged in a plexiform pattern (Figure 29.22). The former cells are mononucleated with vesicular nuclei, abundant clear cytoplasm, and well-defined borders; the syncytiotrophoblastic cells have abundant vacuolated basophilic or amphophilic cytoplasm and several dark nuclei. In some tumors, the plexiform pattern may not be apparent.1 Intermediate trophoblastic cells are present in some cases. Vascular invasion is frequent. The syncytiotrophoblastic cells are immunoreactive for hCG, cytokeratins, human placental lactogen, PLAP, EMA, CD10, CEA, α-inhibin, and GLP-3.
The clinical symptoms and histologic picture of germ cell-derived choriocarcinomas are similar to those of gestational choriocarcinoma; however, the remarkable response to chemotherapy (methotrexate or actinomycin D) associated with the latter tumors does not occur.76 Although gestational choriocarcinomas of the ovary are almost invariably metastatic from uterine or tubal choriocarcinomas, they may occasionally follow an ovarian pregnancy.1,75 The finding of a corpus luteum of pregnancy may be of help in establishing the gestational nature of the tumor. Similarly, the presence of a paternal component by DNA analysis or HLA typing is characteristic of gestational choriocarcinomas.77–79 Rare cases of poorly differentiated carcinomas with choriocarcinomatous differentiation and hCG secretion have been reported.8,80 Unilateral salpingo-oophorectomy followed by combination chemotherapy has resulted in cures or prolonged remissions.
Teratomas
Teratomas are germ cell tumors composed of an array of tissues derived from two or three embryonic layers (ectoderm, mesoderm, and endoderm) in any combination. The great majority of teratomas are benign cystic tumors that contain mature elements and are designated mature cystic teratomas or dermoid cysts. The presence of any immature tissue warrants a diagnosis of IT, which is a potentially malignant neoplasm. Nevertheless, typical dermoid cysts containing very small foci of immature tissue have a benign behavior and should not be reported as grade 1 IT.81 Rarely, teratomas may be predominantly or exclusively composed of endodermal or ectodermal tissues (monodermal teratomas).1 The pathogenesis of teratomas has always excited speculation because of their exotic composition. Cytogenetic analysis, using chromosome-banding techniques, has revealed that ovarian teratomas are parthenogenetic tumors that develop from a single germ cell after its first meiotic division.82 Also, genetic analysis of mature ovarian teratomas has demonstrated genotypic differences between homozygous teratomatous tissue and heterozygous host tissue.83
Mature Teratomas
Clinical Features
Mature teratomas, usually dermoid cysts, represent the most common ovarian neoplasms, accounting for 27–44% of all primary ovarian tumors and over two-thirds of all ovarian tumors in patients under 15 years of age.1,84 Most patients are between 20 and 40 years, but the tumors occur at all ages. Although they usually occur in pure form, dermoid cysts are found grossly within 26% of ITs and in the ovary contralateral to a MGCT in about 5–10% of cases.1,81 Most patients with mature teratomas present the typical signs and symptoms of benign ovarian tumors, but approximately 25% are asymptomatic and the tumors are discovered on routine examination. Radiologically, dermoid cysts are characterized by central areas of very low density enclosed by a ring of increased capsular density.85 Calcified tissues including bone and teeth may be present and facilitate the radiologic diagnosis. Dermoid cysts may undergo torsion or rupture, with acute abdominal symptoms, or hemoperitoneum. Leakage of the cyst contents into the abdominal cavity results in granulomatous peritonitis, which may mimic tuberculosis or metastatic carcinoma.86 Peritoneal melanosis may also occur. Autoimmune hemolytic anemia has been reported in some cases. Mature solid teratomas may occasionally produce mature peritoneal glial implants (grade 0), which have an excellent prognosis.87
Although mature teratomas are benign tumors, malignant transformation may occur in approximately 1–2% of cases, usually in postmenopausal women (mean age, 51–62 years). Almost any component may become malignant, but squamous cell carcinoma accounts for 80% of the cases.1,88
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree