and Natalia Buza1
(1)
Department of Pathology, Yale University School of Medicine, New Haven, CT, USA
Keywords
Mesenchymal neoplasmsLeiomyomaLeiomyosarcomaSmooth muscle tumor of uncertain malignant potentialEndometrial stromal tumorsMullerian adenosarcomaMalignant mixed Mullerian tumorUterine tumor resembling ovarian sex-cord tumorPerivascular epithelioid cell tumorIntroduction
Uterine mesenchymal neoplasms are generally divided into smooth muscle tumors, endometrial stromal tumors, perivascular epithelioid cell tumors, and other mesenchymal tumors of both homologous and heterologous tissue types. In addition, tumors with mixed epithelial and mesenchymal components are also included in this chapter (Table 5.1) [1]. While benign leiomyomas are very common, uterine leiomyosarcoma and endometrial stromal tumors are rare, frequently unexpected lesions at the time of frozen section. The primary function of intraoperative consultation of uterine mesenchymal lesions in a hysterectomy specimen is to rule out malignancy. Careful gross inspection with attention to tumor border infiltration, color, and texture is crucial for adequate sampling of a uterine mesenchymal neoplasm for frozen section evaluation.
Table 5.1
2014 World Health Organization classification of uterine mesenchymal tumors. Data from Kurman et al. [1]
Major heading | Subheading | Subtype | Variant | Variant | Variant |
|---|---|---|---|---|---|
Endometrial stromal tumors | Endometrial stromal nodule | With smooth muscle differentiation | |||
Endometrial stromal sarcoma | Low-grade endometrial stromal sarcoma | With smooth muscle differentiation | |||
High-grade endometrial stromal sarcoma | |||||
Mixed epithelial and stromal tumors | Adenomyoma | Atypical polypoid adenomyoma (APA) | |||
Adenosarcoma | With sarcomatous overgrowth | ||||
Malignant mixed Mullerian tumor (carcinosarcoma) | |||||
Smooth muscle tumors | Spindle smooth muscle tumor | Leiomyoma | Mitotically active leiomyoma | Leiomyoma with bizarre nuclei | Cellular leiomyoma |
Leiomyosarcoma | |||||
Epithelioid smooth muscle tumor | Epithelioid leiomyoma | Plexiform tumorlet | |||
Epithelioid leiomyosarcoma | |||||
Myxoid smooth muscle tumor | Myxoid leiomyoma | ||||
Myxoid leiomyosarcoma | |||||
Smooth muscle tumor of uncertain malignant potential (STUMP) | |||||
Smooth muscle tumor with unusual growth pattern or clinical behavior | Diffuse leiomyomatosis | ||||
Dissecting leiomyoma | |||||
Intravenous leiomyomatosis | |||||
Metastasizing leiomyoma | |||||
Perivascular epithelioid cell tumors (PEComa) | Conventional PEComa | PEComatosis | |||
Other mesenchymal and miscellaneous tumors | Homologous sarcoma | Angiosarcoma | |||
Fibrosarcoma | |||||
Neurogenic sarcoma | |||||
Heterologous sarcoma | Rhabdomyosarcoma | ||||
Alveolar soft part sarcoma | |||||
Rhabdoid tumor | |||||
Proximal epithelioid sarcoma | |||||
Undifferentiated uterine sarcoma | |||||
Uterine tumor resembling ovarian sex-cord tumor | |||||
Inflammatory myofibroblastic tumor | |||||
Adenomatoid tumor |
Smooth Muscle Tumors
Smooth muscle tumors of the uterus (see Table 5.1) [1] are categorized into leiomyoma, leiomyosarcoma, smooth muscle tumor of uncertain malignant potential (STUMP), and subsets of histologically mature smooth muscle tumors with either unusual growth patterns or unique clinical behavior (benign metastasizing leiomyoma, intravenous leiomyomatosis, diffuse peritoneal leiomyomatosis, peritoneal parasitic leiomyoma, uterine leiomyomatosis, and dissecting leiomyoma/cotyledonoid leiomyoma).
Conventional Leiomyoma
Clinical features
The most common uterine mesenchymal tumor seen in over 2/3 of hysterectomy specimens.
Vaginal bleeding and pelvic pain or pressure are common clinical presentations.
Gross pathology (Fig. 5.1)
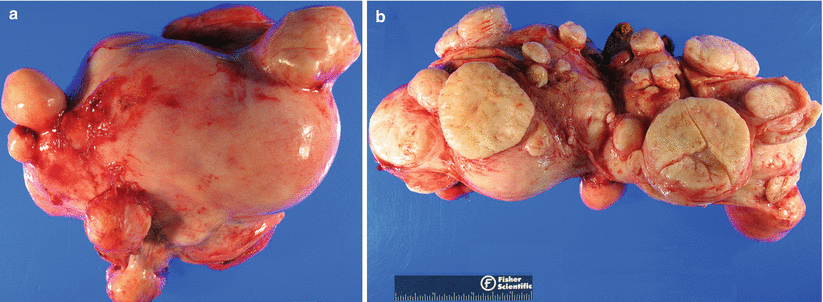
Fig. 5.1
Various gross presentations of uterine leiomyomas. Note the well-circumscribed round to oval nodules (a) with bulging, solid, and whorled cut surfaces (b)
Round to oval nodule involving submucosa, myometrium, or subserosa with multiple lesions seen in 2/3 of the cases
Well-circumscribed, rubbery firm, and solid masses with bulging and whorled cut surface
Frequent degenerative changes leading to various colors (red, brown, yellow, and hemorrhagic) and texture (edematous, fleshy to obviously necrotic), particularly in a large tumor (Fig. 5.2) and may be quite alarming for possible malignancy (Fig. 5.3)
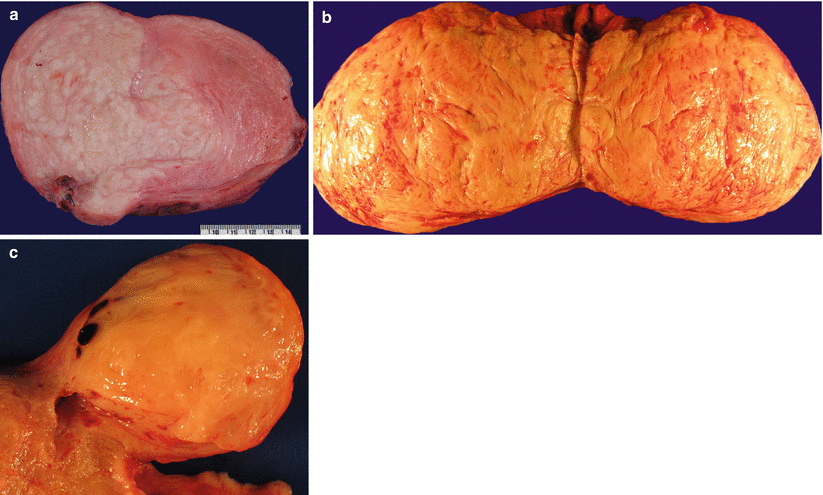
Fig. 5.2
Leiomyomas with degenerative changes. Note the varying colors (white, brown, or yellow) and texture (firm, fibrotic, or soft, edematous) (a–c)
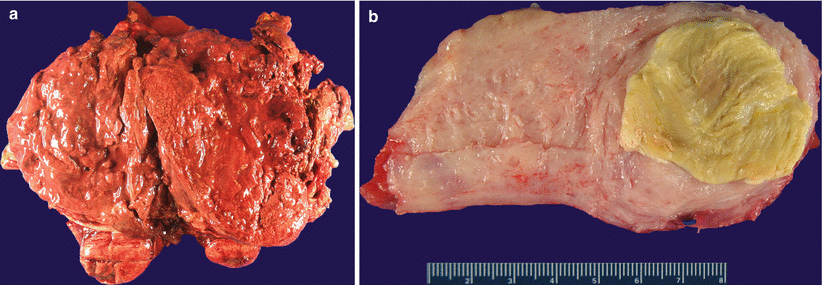
Fig. 5.3
Leiomyomas with a gross appearance suspicious for malignancy. Note the large areas of necrosis and hemorrhage (a, b)
Microscopic features
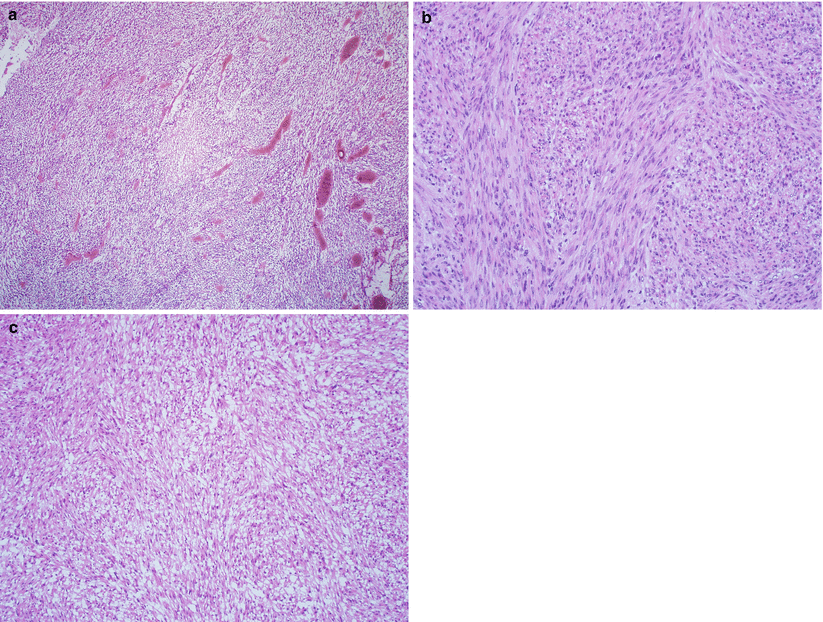
Nodular proliferation of fascicles or bundles of mature, elongated smooth muscle cells with eosinophilic cytoplasm, and a centrally located cigar-shaped nucleus (Fig. 5.4).
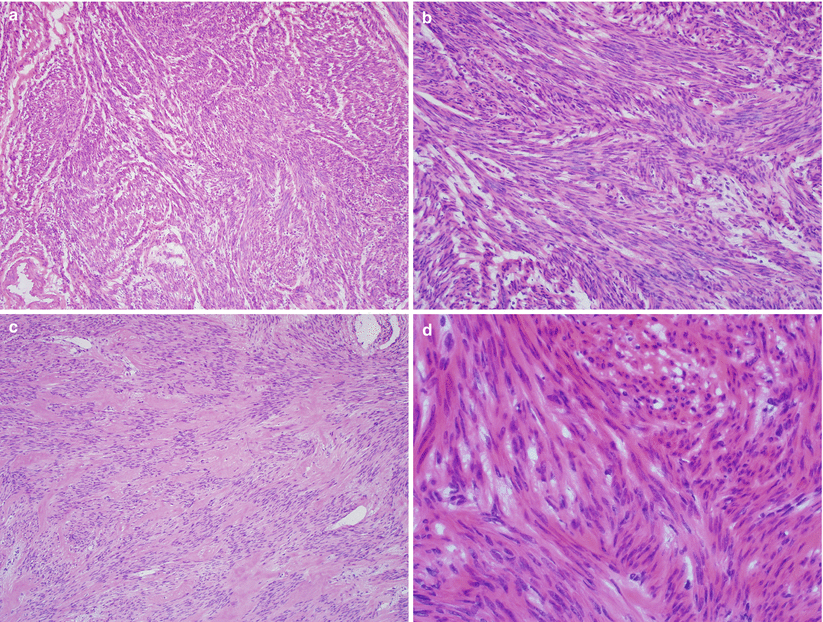
Fig. 5.4
Conventional leiomyoma. Note the mature smooth muscle proliferation in fascicles and bundles with variable stromal hyalinization (a–c) and characteristic cigar-shaped nuclei of the tumor cells (d)
Rich vasculature with characteristic gaping, thick-walled large caliber vessels.
Intratumoral hyalinization is common and may be extensive in some cases.
Secondary changes
Infarction-type necrosis with typical zonal configuration (necrotic smooth muscle cells are surrounded by a rim of granulation or fibrous tissue with varying degrees of hyalinization).
Intratumoral hemorrhage or red degeneration (commonly seen after hormonal treatment, with concurrent pregnancy or secondary to uterine artery embolization for symptomatic leiomyomas) (Fig. 5.5).
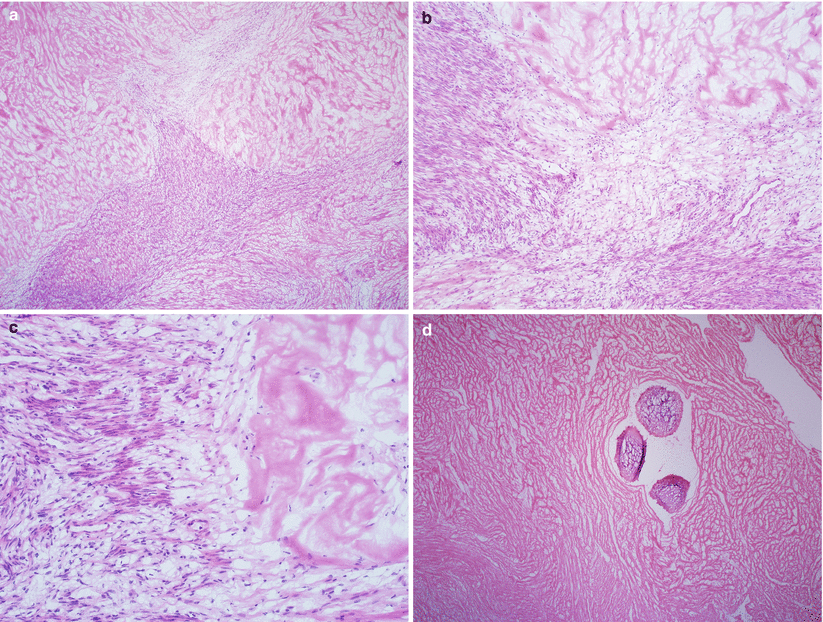
Fig. 5.5
Leiomyoma with hemorrhagic infarction due to therapeutic embolization. Note the characteristic zonal arrangement of the infarcted area, with gradual transition to granulation tissue and to viable smooth muscle cells (a–c). Intravascular foreign material—used for embolization—is apparent (d)
Leiomyoma Variants
Cellular leiomyoma [2]
Grossly fleshy tumor nodules.
Histologically hypercellular compared to adjacent myometrium, some approaching the cellularity of an endometrial stromal tumor (Fig. 5.6).
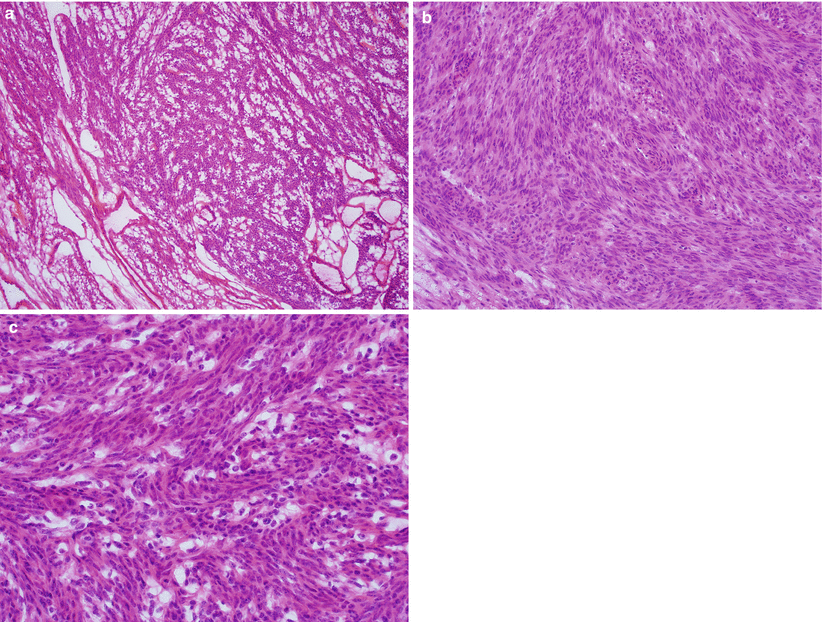
Fig. 5.6
Cellular leiomyoma. Note the marked hypercellularity compared to the adjacent myometrium (a) and lack of significant nuclear atypia and mitotic activity (b, c)
Cleft-like or gaping, thick-walled vascular spaces are common.
Tumor border may show focal irregular extension into the adjacent myometrium.
Differential diagnosis
Low-grade endometrial stromal sarcoma
Smooth muscle tumor of uncertain malignant potential (STUMP)
Leiomyosarcoma
Diagnostic pitfalls/key intraoperative consultation issues
The presence of fascicular growth pattern and large, gaping vessels favors a leiomyoma over an endometrial stromal tumor.
In contrast to STUMP and leiomyosarcoma, cellular leiomyoma has minimal mitotic activity and absence of coagulative tumor cell necrosis and cytological atypia.
When in doubt, interpretation of “smooth muscle tumor, classification is deferred for permanent sections” is recommended for conservative surgical management.
Leiomyoma with bizarre nuclei or symplastic leiomyoma [1, 3]
The salient microscopic finding is the focal or diffuse presence of scattered, large atypical smooth muscle cells with abundant eosinophilic cytoplasm and bizarre nuclear shapes and multinucleation.
The bizarre tumor cells have hyperchromatic nuclei with frequent intranuclear inclusions.
Low mitotic count of less than 5 per 10 high-power fields (HPF)
Differential diagnosis (Table 5.2)
Table 5.2
Uterine smooth muscle tumors with spindled morphology (excluding epithelioid and myxoid variants)
Coagulative tumor cell necrosisa
Moderate to severe atypia
Mitoses/10 HPF
Interpretation
None
None
<5
Leiomyoma
5 to 14
Mitotically active leiomyoma
≥15
STUMP
Focal or diffuse
<5
Leiomyoma with bizarre nuclei
5–9
STUMP
≥10
Leiomyosarcoma
Present
None
<10
STUMP
≥10
Leiomyosarcoma
Focal or diffuse
Any
Leiomyosarcoma
Uncertain
None
Any
STUMP
Leiomyosarcoma
Diagnostic pitfalls/key intraoperative consultation issues
In contrast to leiomyosarcoma, leiomyoma with bizarre nuclei lacks diffuse moderate to severe cytological atypia, mitotic activity, and coagulative tumor cell necrosis.
Chromatin condensation and fragmentation should not be misinterpreted as mitoses or atypical mitotic figures.
When in doubt, interpretation of “smooth muscle tumor with atypical features, classification is deferred for permanent sections” may be communicated for conservative surgical management.
Mitotically active leiomyoma
The tumor may be associated with high progestin levels (secretory phase, pregnancy, or exogenous hormones) [1, 4, 5].
Histologically typical leiomyoma or cellular leiomyoma with 4–15 mitoses per 10 HPF.
Significant nuclear atypia and coagulative tumor cell necrosis are absent.
Diagnostic pitfalls/key intraoperative consultation issues
In contrast to leiomyosarcoma, mitotically active leiomyoma lacks diffuse cytological atypia and coagulative tumor cell necrosis.
When in doubt, interpretation of “smooth muscle tumor with increased mitotic activity, classification is deferred for permanent sections” is recommended for conservative surgical management.
Apoplectic leiomyoma (Fig. 5.7) [6, 7]
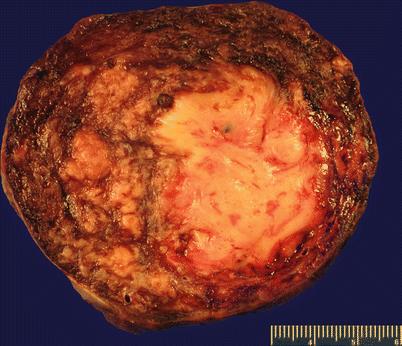
Fig. 5.7
Apoplectic leiomyoma. Note the presence of diffuse hemorrhage
Patient with history of progestin treatment for symptomatic leiomyoma.
Grossly typical leiomyoma with foci of hemorrhage.
Histologically cellular leiomyoma with areas of hemorrhage and necrosis surrounded by zones of cellular smooth muscle proliferation that may have increased mitotic activity (Fig. 5.8), but no more than 8 per 10 HPF [7].
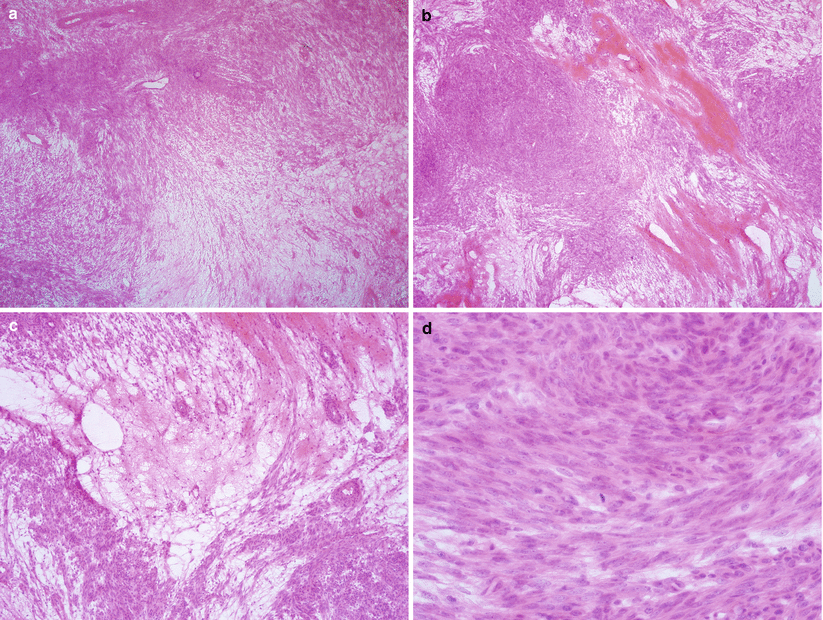
Fig. 5.8
Apoplectic leiomyoma. Cellular leiomyoma with hemorrhage, edema (a–c), and increased mitotic activity (d)
Granulation tissue, hyalinization, and myxoid degeneration may occur.
Differential diagnosis
Leiomyosarcoma
Diagnostic pitfalls/key intraoperative consultation issues
The tumor lacks nuclear atypia and coagulative tumor cell necrosis.
When in doubt, interpretation of “smooth muscle tumor with atypical features, classification is deferred for permanent sections” is recommended for conservative surgical management.
Myxoid leiomyoma (Fig. 5.9)
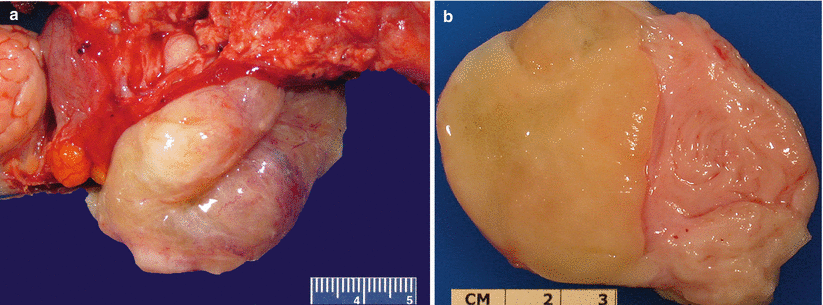
Fig. 5.9
Myxoid leiomyoma. Note the gelatinous gross appearance in these two examples (a, b)
Rare variant of leiomyoma that may be associated with a concurrent pregnancy.
Marked hypocellularity with small spindle or stellate cells embedded in a myxoid matrix [8].
Some myxoid leiomyomas may have an infiltrative border.
Nuclear atypia, coagulative tumor cell necrosis, and mitotic activity are absent (Fig. 5.10).
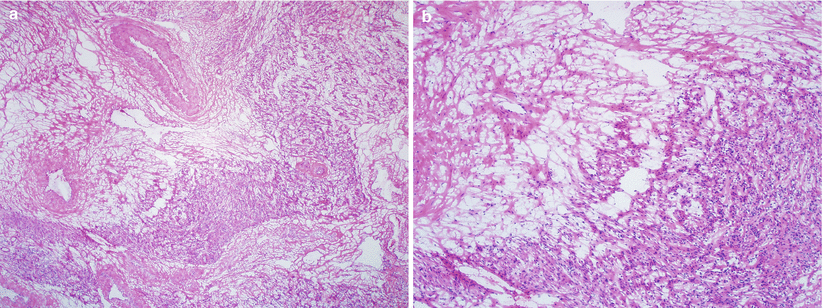
Fig. 5.10
Myxoid leiomyoma. Note the myxoid hypocellular areas in transition to a conventional leiomyoma area (a, b)
Differential diagnosis
Leiomyosarcoma
Diagnostic pitfalls/key intraoperative consultation issues
For any myxoid tumor with an infiltrative tumor border or any level of noticeable mitotic activity, an interpretation of “myxoid smooth muscle tumor cannot rule out malignancy or defer to permanent section” is recommended for conservative surgical management.
Epithelioid leiomyoma (leiomyoblastoma) [9] (Fig. 5.11)
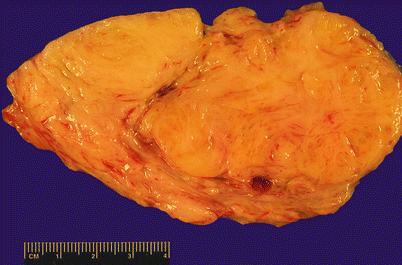
Fig. 5.11
Epithelioid leiomyoma presents as tan nodular lesions
Grossly indistinguishable from conventional leiomyoma.
Histological proliferation of polygonal to round cells in sheets, nests, or cords (Fig. 5.12).
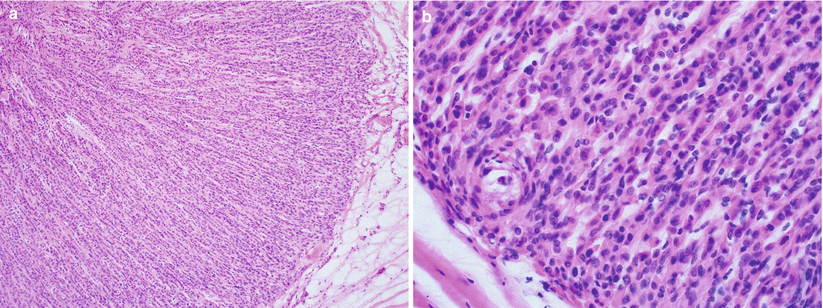
Fig. 5.12
Epithelioid leiomyoma. Note the polygonal to round, uniform epithelioid cells with eosinophilic cytoplasm arranged in cords (a, b)
Admixture with conventional spindled smooth muscle cells is common.
Tumor cells have eosinophilic or clear cytoplasm.
Absence of cytological atypia, necrosis, and mitotic activity.
Its microscopic variant, plexiform tumorlet is generally an incidental microscopic finding [10], most often solitary but may also be multiple.
Diffuse leiomyomatosis [11]
Presence of numerous cellular leiomyomatous nodules merging into each other and diffusely involving the myometrium, leading to diffuse and symmetrical enlargement of the uterus.
Histologically the tumor is identical to conventional leiomyoma.
Lipoleiomyoma
Grossly yellow to tan nodular lesion (Fig. 5.13).
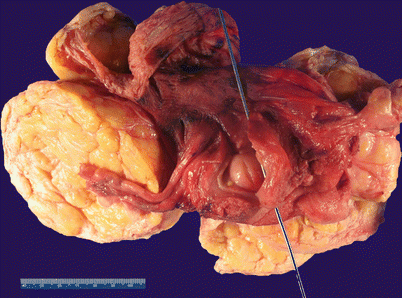
Fig. 5.13
Lipoleiomyoma. Note the presence of well-circumscribed, yellow-tan intramural nodules
Leiomyoma with intermixed mature adipocytes (Fig. 5.14).
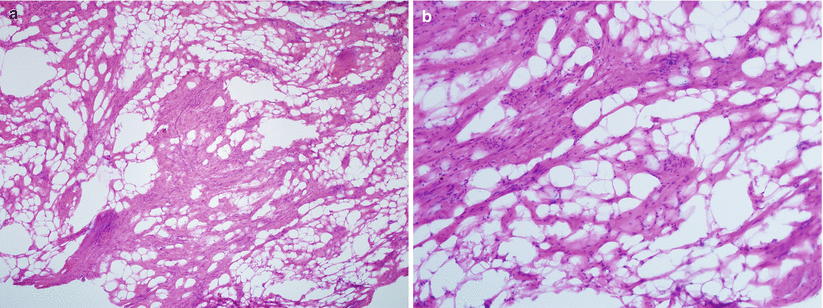
Fig. 5.14
Lipoleiomyoma. Note the variable amount of mature adipocytes admixed within bundles of smooth muscle cells (a, b)
Focal chondroid or other heterologous components may be seen [12].
Differential diagnosis includes adenomatoid tumor. However, separation of the two benign entities is not crucial at the time of frozen section diagnosis.
Hydropic leiomyoma (Fig. 5.15)
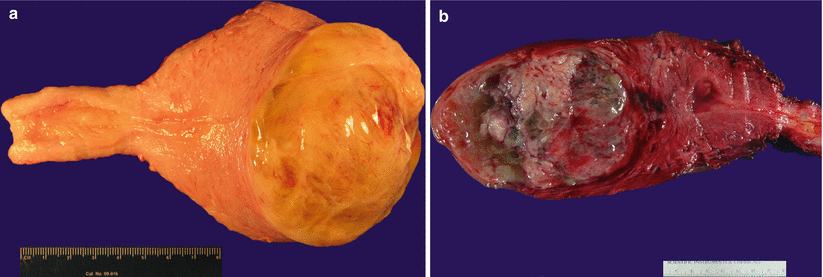
Fig. 5.15
Hydropic leiomyoma. Note semitransparent edematous nodules (a) with focal cystic changes (b)
Leiomyoma with focal or zonal edema [13].
Edema separates bundles or nodules of smooth muscle. Pseudocyst formation may be seen within the hydropic area.
Differential diagnosis
Leiomyosarcoma
Diagnostic pitfalls/key intraoperative consultation issues
In contrast to leiomyosarcoma, hydropic leiomyoma has a zonal hydropic change, non-infiltrative border, and benign cytological features.
Leiomyomas with Unusual Growth Pattern and/or Clinical Behavior
Intravenous leiomyomatosis (IVL) [14, 15] [16]
Clinical symptoms are similar to conventional leiomyoma.
Some patients have history of multiple myomectomies.
Grossly multiple, irregular myometrial nodular or wormlike lesions, some of which may be grossly intravascular.
Tumor may extend into parametrial or pelvic vessels and occasionally further into the vena cava and right atrium and ventricle (Fig. 5.16).
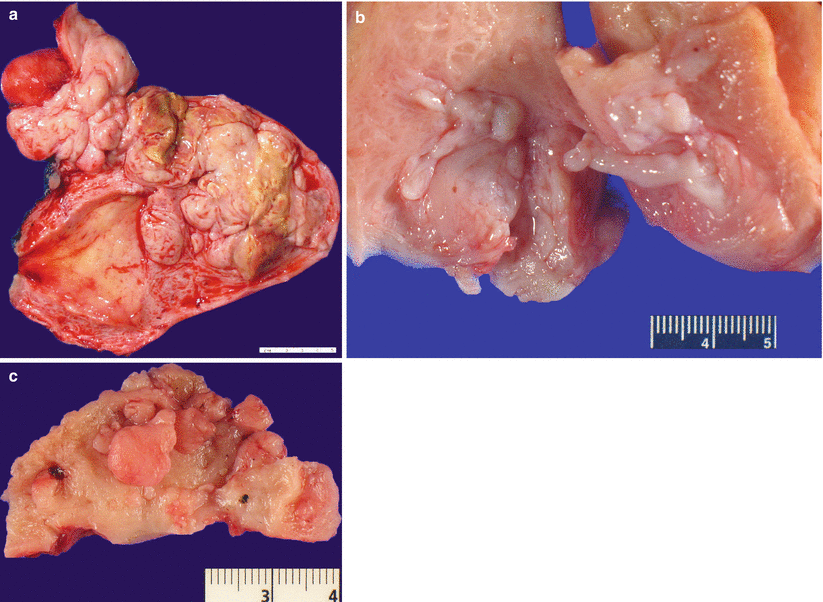
Fig. 5.16
Intravenous leiomyomatosis. Note the presence of multiple, irregular, nodular myometrial, and wormlike intravascular lesions (a–c). Note the extrauterine extension of the tumor (a, upper left corner)
Microscopically proliferation of serpentine smooth muscle bundles tracking and plugging vasculature at the tumor periphery and beyond (Fig. 5.17a, b).
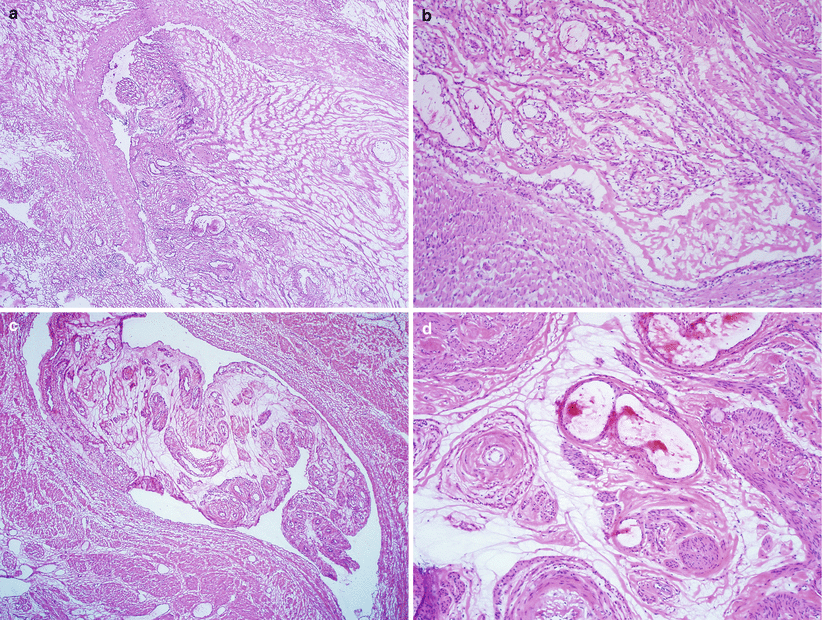
Fig. 5.17
Intravenous leiomyomatosis (IVL). Note the smooth muscle bundles tracking and plugging large caliber vasculature (a, b) and the presence of tissue molding, clefting, hyalinization, and vascularization along with diminished smooth muscle cells (c, d)
Intravascular tumor plugs are
Differential diagnosis (Table 5.3)
Table 5.3
Frozen section diagnosis of mesenchymal tumors with intravascular growth pattern
Intravenous leiomyomatosis
Low-grade endometrial stromal sarcoma
Leiomyosarcoma
Patient age
Any age
Mostly <50 years of age
Mostly > 40 years of age
Gross pathology
Multinodular, white, rubbery masses with surrounding wormlike vascular plugging
Infiltrative endo-myometrial soft tan mass with wormlike vascular plugging
Large mass with irregular border and fleshy, necrotic cut surface, may show intravascular mass lesions
Growth pattern
Intravenous growth of leiomyoma, may be cellular; often hyalinized or shows abundant prominent thick-walled vessels
Infiltrative border and tonguelike tumor growth involving adjacent myometrium and lymphovascular spaces
Starburst hyaline plaques and sex-cord differentiation may be seen
Spindle smooth muscle cells with coagulative tumor cell necrosis and hemorrhage
Cytological features
Bland, elongated spindle smooth muscle cells
Monotonous small oval to short spindle cells with scant cytoplasm
Spindle smooth muscle cells with diffuse moderate to severe nuclear atypia
Mitotic activity
None
Low
Frequent (>10 mitoses/10 HPF)
Intratumoral vasculature
Large gaping vessels
Evenly distributed small arterioles
Variably sized vasculature
Extrauterine disease
Often present involving venous structures with thin wormlike plugging
Often seen involving lymphatics with a wormlike plugging appearance
When present, marked expansion of existing vasculature by tumor nodules
Conventional leiomyomas with irregular tumor borders
Leiomyoma with focal vascular invasion
Entrapped arteries within myometrial veins (vessel within vessel phenomenon)
Low-grade endometrial stromal sarcoma
Intravenous growth of leiomyosarcoma
Diagnostic pitfalls/key intraoperative consultation issues
True intravascular growth of smooth muscle separates IVL from its benign mimics.
IVL lacks cytological features and characteristic vasculature of low-grade endometrial stromal sarcoma.
When in doubt, careful examination of the gross specimen including extrauterine vasculature and additional frozen sections should be performed.
Diffuse peritoneal leiomyomatosis (disseminated peritoneal leiomyomatosis) [18–20]
Patients of reproductive age and over 2/3 of them with a concurrent pregnancy.
May be an incidental finding at the time of C-section or postpartum tubal ligation.
Grossly appears as multiple peritoneal nodules of less than 1 cm in size (Fig. 5.18).
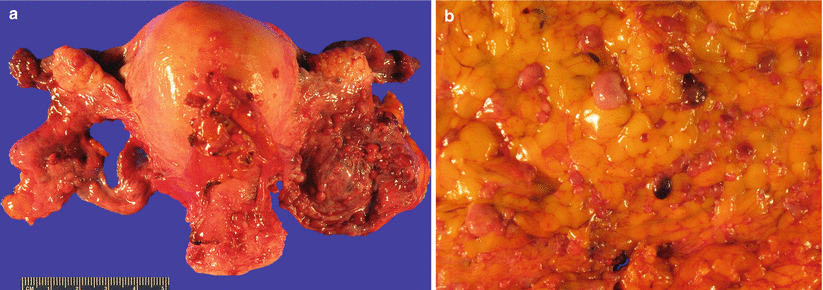
Fig. 5.18
Diffuse peritoneal leiomyomatosis. Note the presence of multiple peritoneal nodules of less than 1 cm on the surface of broad ligaments (a) and omentum (b)
Mature smooth muscle cell proliferation with occasional mitoses (less than 3 per 10 high-power fields) without significant cytological atypia and coagulative tumor cell necrosis (Fig. 5.19).
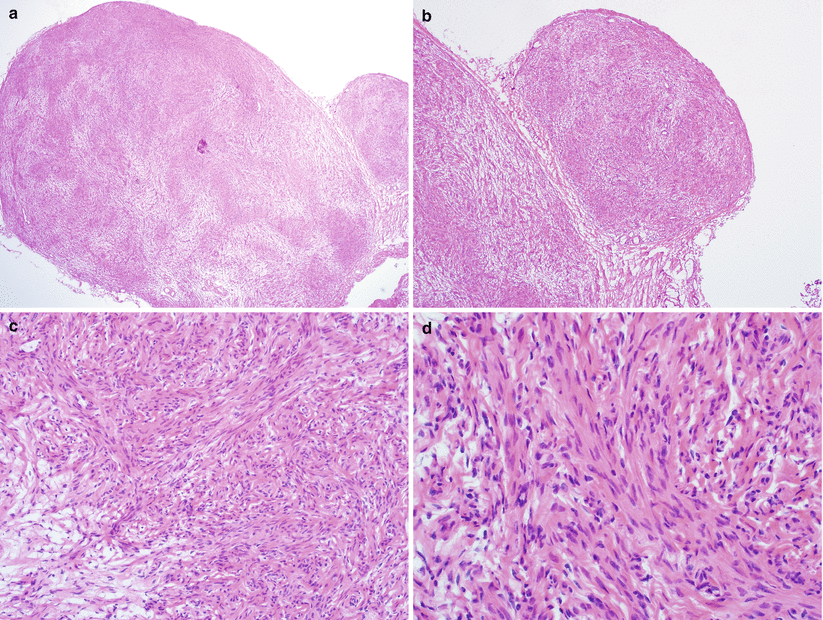
Fig. 5.19
Diffuse peritoneal leiomyomatosis. Nodular proliferation of mature smooth muscle cells (a, b) without cytological atypia, mitotic activity, or tumor cell necrosis (c, d)
Generally a self-limiting disease with regression after pregnancy. Recurrence may be seen in subsequent pregnancies, however, and malignant transformation to leiomyosarcoma has been reported in rare cases.
Differential diagnosis
Leiomyosarcoma
Diagnostic pitfalls/key intraoperative consultation issues
In contrast to leiomyosarcoma, diffuse peritoneal leiomyomatosis lacks cytological atypia and coagulative tumor cell necrosis.
Benign metastasizing leiomyoma [21]
Conventional uterine leiomyoma with concurrent or subsequent (post-myomectomy) extrauterine tumors of similar histology
Frequent involvement of lung and rarely retroperitoneal and mediastinal lymph nodes, bone, and soft tissue
Differential diagnosis
Leiomyosarcoma
Diagnostic pitfalls/key intraoperative consultation issues
In contrast to leiomyosarcoma, metastasizing leiomyoma lacks diffuse cytological atypia and coagulative tumor cell necrosis.
Dissecting leiomyoma [22, 23]
Grossly lobulated myometrial lesion with ill-defined tumor border.
Histologically dissecting growth of bundles of mature smooth muscle cells protruding and splitting uterine myometrium.
In extremely rare cases, the dissecting smooth muscle bundles may extend into the broad ligament, forming a large mushroomlike mass (cotyledonoid variant).
The gross appearance may be quite alarming at the time of frozen section diagnosis. Leiomyosarcoma should be ruled out by careful microscopic examination.
Leiomyosarcoma [4] [1, 24]
Clinical features
Most common uterine sarcoma.
Adult patients over 40 years of age, with a mean age of 55 years.
May present with sudden uterine tumor growth in patients not on hormone replacement [25].
Other clinical symptoms include vaginal bleeding, pelvic mass, and pelvic pain or pressure.
Gross pathology (Fig. 5.20)
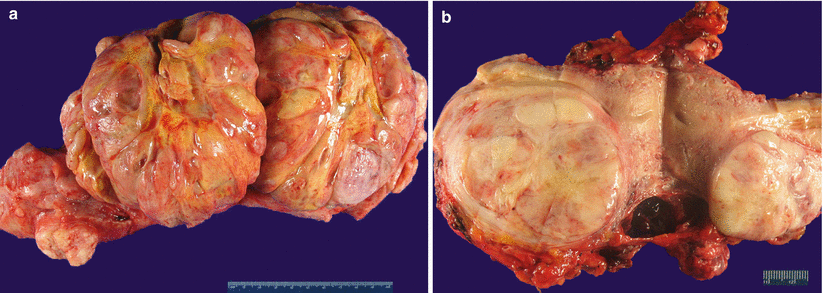
Fig. 5.20
Leiomyosarcoma (spindle cell). Large dominant intramural mass with a fleshy cut surface showing hemorrhage and necrosis (a, b)
Generally solitary large mass (average size of 10 cm) or the largest mass among multiple conventional leiomyomas.
Fleshy, soft, and bulging cut surface with varying colors and textures.
Infiltrative tumor border may be grossly apparent.
Necrosis and hemorrhage are common.
Microscopic features (see Table 5.2)
Hypercellular spindle cell proliferation forming disorganized fascicles and nodules (Fig. 5.21).