and Natalia Buza1
(1)
Department of Pathology, Yale University School of Medicine, New Haven, CT, USA
Keywords
Malignant mesotheliomaWell-differentiated papillary mesotheliomaGastrointestinal stromal tumorDesmoplastic small round cell tumorPseudomyxoma peritoneiReactive mesothelial proliferation/hyperplasiaEndosalpingiosisEndocervicosisPeritoneal inclusion cystIntroduction
Peritoneal biopsies are often performed and sent for frozen section evaluation during gynecologic surgeries. Intraoperative assessment of peritoneal surfaces is an integral part of surgical staging procedure for gynecologic malignancies. Peritoneal/omental nodules are usually more easily accessible at the beginning of the surgical procedure than other pelvic/gynecologic organs, and the frozen section diagnosis provides guidance for the type and extent of the surgery. While the peritoneum is often involved by various benign and malignant tumors originating from the gynecologic tract, tumors of other histogenetic origins and reactive conditions also occur and may pose a significant diagnostic challenge on frozen section.
Mullerian Epithelial Tumors
Serous tumors—serous borderline (atypical proliferative) tumor, low-grade and high-grade serous carcinoma—may arise from the peritoneum as a primary site, and their histological features and diagnostic criteria are essentially identical to those of ovarian origin. (See Chap. 8—for detailed clinicopathologic features.)
Primary ovarian serous tumors often show peritoneal involvement—implants of serous borderline (atypical proliferative) tumor or carcinoma.
The primary tumor site—ovarian versus peritoneal—often cannot be determined based on a small peritoneal biopsy alone.
There is no significant difference in intraoperative surgical management between primary ovarian and peritoneal serous tumors.
Recognition of atypical/neoplastic nature of the process and serous (Mullerian) histological subtype are the most important for clinical management at the time of frozen section.
Various nonneoplastic/reactive and neoplastic mimics exist and will be discussed in detail in this chapter.
Mesothelial Tumors
Malignant Mesothelioma
Clinical features
Majority of patients are male with history of occupational asbestos exposure [1].
Most common location is pleura, while peritoneal mesothelioma represents only 10–30 % of cases [1, 2].
Female patients with peritoneal mesothelioma less often have history of asbestos exposure and present at a mean age of 47.4 years (range 17–92 years) [3].
Gross pathology
Diffuse involvement of peritoneal surfaces by nodules, plaques, or papillary excrescences [3]
Microscopic features (Fig. 13.1)
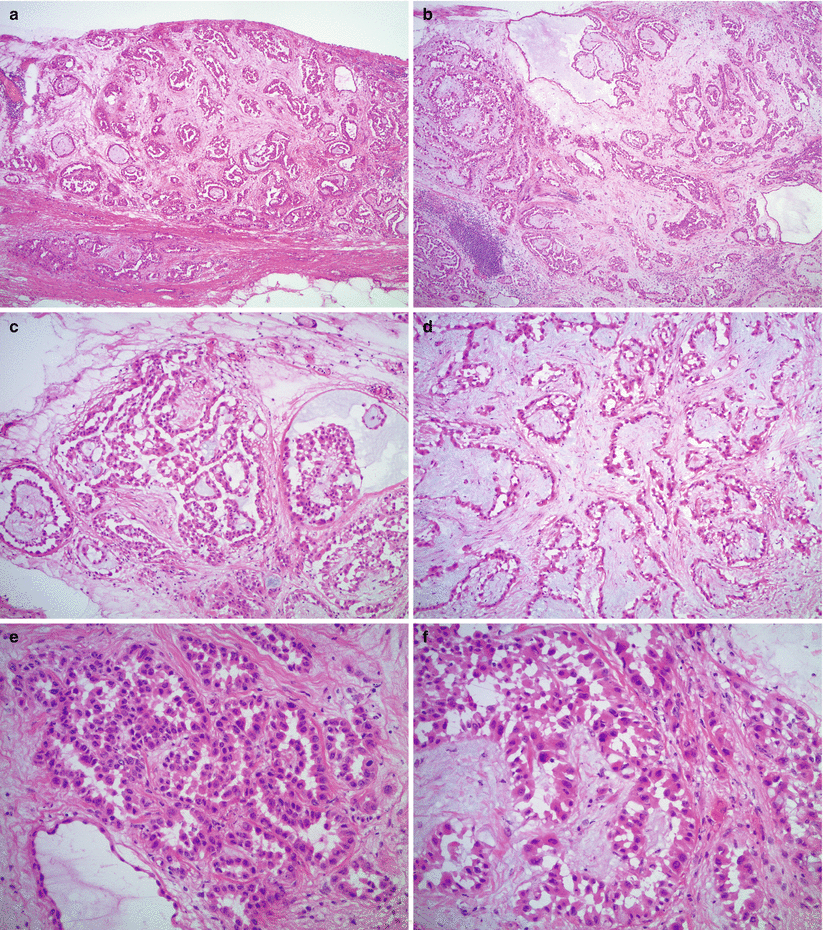
Fig. 13.1
Malignant mesothelioma. Note the plaque-like growth on the peritoneal surface (a) with papillary (b, c) and reticular (d) architecture. Pale basophilic intraluminal material (b, c) and myxoid stroma (d) may be seen. The cells show mild to moderate atypia and “hobnail” or “cobblestone” appearance (e, f)
Epithelioid cells arranged in tubular, papillary, or solid architectural patterns—often multiple patterns within the same tumor.
Compressed tubular structures may resemble a reticular pattern.
Polygonal or round tumor cells with moderate amount of eosinophilic cytoplasm.
“Hobnail” and “cobblestone” appearance may be seen.
Mild to moderate atypia in most cases; rarely severe nuclear atypia.
Mitotic activity is usually low; less than 5/10 high-power field in most tumors [3].
Pale basophilic material may be seen in the lumen of tubules or within the cytoplasm.
Stromal infiltration is usually present; desmoplastic response may be seen.
Stroma may be hyalinized or less often myxoid.
Psammomatous calcifications may be present.
Rare histological variants—sarcomatoid, deciduoid, signet-ring cell, and multicystic—are also known to occur.
Differential diagnosis
Mullerian serous tumors—high-grade and low-grade serous carcinoma, serous borderline (atypical proliferative) tumor
Metastatic carcinomas from other primaries
Well-differentiated papillary mesothelioma
Reactive mesothelial hyperplasia
Diagnostic pitfalls/key intraoperative consultation issues
Malignant mesothelioma in women is much less common than Mullerian serous tumors.
The surgeon should be inquired about the intraoperative gross appearance of the lesion: a small, solitary nodule favors well-differentiated papillary mesothelioma.
The biopsy sent for frozen section is often small, and the histological features may be misleading without knowledge of the clinical context and gross appearance/extent of disease.
The mitotic activity is usually higher and the nuclear pleomorphism is more severe in high-grade serous carcinomas, compared with malignant mesothelioma.
Intraluminal and intracytoplasmic basophilic material may be seen in mesotheliomas, but it is not a feature of Mullerian serous tumors.
Desmoplastic and/or myxoid stromal reaction and intraluminal basophilic material may mimic metastatic adenocarcinoma, especially from gastrointestinal or pancreatobiliary primaries.
Definitive diagnosis of malignant mesothelioma on frozen section (i.e., on morphology alone, without ancillary studies) may be very challenging; however, based on the clinical and gross features and microscopic characteristics of tumor, the possibility of mesothelioma should be raised.
Well-Differentiated Papillary Mesothelioma
Clinical features
Gross pathology
Usually presents as a solitary or multiple small nodule(s), less than 2 cm in size [6]
Microscopic features (Fig. 13.2)
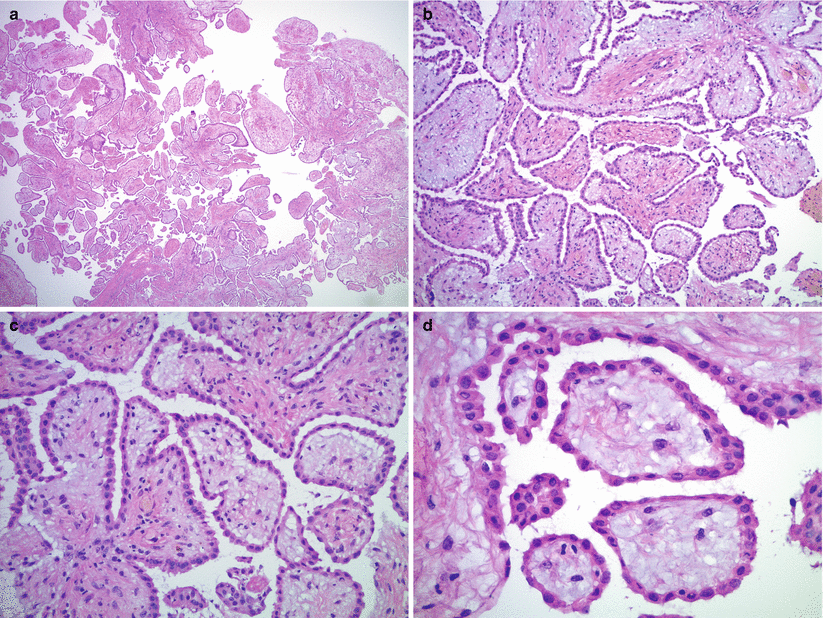
Fig. 13.2
Well-differentiated papillary mesothelioma. The tumor has papillary architecture on low magnification (a, b) without epithelial stratification or tufting. The papillae are lined by flat or cuboidal, bland mesothelial cells (c, d)
Papillary or tubulo-papillary architecture.
Single layer of flat or cuboidal, bland mesothelial cells.
No significant atypia or mitotic activity.
Lack of stromal infiltration.
Psammoma bodies may be seen.
Differential diagnosis
Malignant mesothelioma
Mullerian serous tumors—low-grade serous carcinoma and serous borderline (atypical proliferative) tumor
Diagnostic pitfalls/key intraoperative consultation issues
Well-differentiated papillary mesothelioma typically shows a benign clinical course.
Intraoperative gross appearance of the lesion is very helpful: malignant mesothelioma is typically diffuse, nodular, or plaque-like, whereas well-differentiated papillary mesothelioma presents as a small, solitary nodule.
Significant nuclear atypia and apparent mitotic activity are in favor of malignant mesothelioma.
Stromal invasion is typically present in malignant mesothelioma but not in well-differentiated papillary mesothelioma.
Low-grade serous tumors—low-grade carcinoma and serous borderline (atypical proliferative) tumor—typically show more pronounced epithelial proliferation, multiple cell layers, and tufting, compared with well-differentiated papillary mesothelioma.
Mesenchymal Tumors
Leiomyomatosis Peritonealis Disseminata
Rare, benign lesion
Multiple small nodules on the peritoneal surfaces and omentum
Composed of bland smooth muscle cells, histologically similar to uterine leiomyomas [see Chap. 5]
No significant nuclear atypia or mitotic activity
Differential diagnosis: gastrointestinal stromal tumor, leiomyosarcoma; other benign and malignant spindle cell proliferations
See Chap. 5 for detailed histological features and differential diagnosis of smooth muscle tumors.
Gastrointestinal Stromal Tumor (GIST)
Clinical features
Gastrointestinal stromal tumors may present—primarily or on recurrence—as an omental, mesenteric, or retroperitoneal mass [9–11].
Most common in middle-aged adults; median age of patients with omental presentation is around 60 years [9].
Patients may present with abdominal mass/pain or it may be an incidental finding during surgery.
Gross pathology (Fig. 13.3)
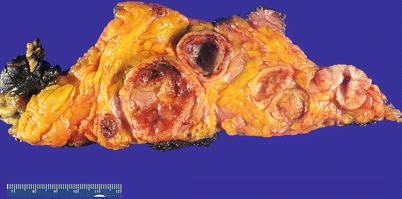
Fig. 13.3
Gastrointestinal stromal tumor (GIST) presenting as an omental mass, forming multiple solid and cystic nodules within the fibroadipose tissue
Single or multiple masses, with a gray-tan or yellowish, predominantly solid cut surface.
May be attached to stomach or small intestine or there may not be evidence of gastrointestinal tract involvement.
Median tumor size is 14 cm [9].
Necrosis, hemorrhage, or cystic change may be seen.
Microscopic features (Fig. 13.4)
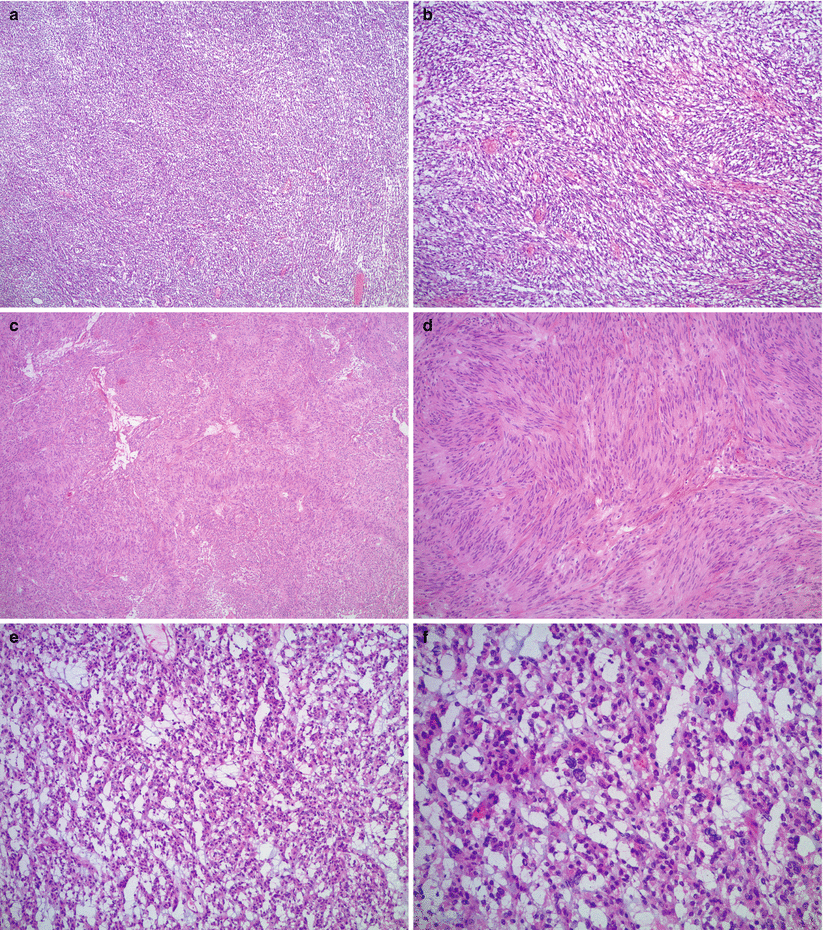
Fig. 13.4
Gastrointestinal stromal tumor (GIST). The tumor most often shows a spindle cell pattern (a, b), occasionally with nuclear palisading (c, d). Epithelioid GIST may have myxoid change and significant nuclear atypia (e, f), mimicking carcinoma
The most common histological pattern is spindle cell, followed by epithelioid and mixed spindle and epithelioid patterns.
Significant nuclear pleomorphism is relatively uncommon, more often seen in epithelioid tumors.
Cellularity is variable.
Nuclear palisading and perinuclear vacuoles may be seen.
Mitotic activity is highly variable.
Myxoid change may be seen.
Necrosis and hemorrhage may be present.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


