Upper and Lower Gastrointestinal Endoscopy
Jeffrey L. Ponsky
Jonathan P. Pearl
Introduction
Since the introduction of flexible endoscopy five decades ago, surgeons have played a major role in its practice. Many of the advances in endoscopy were developed by surgeons, and endoscopy continues to be the mainstay of diagnosis and treatment of common gastrointestinal disorders.
We have entered a new era in endoscopic therapy. The prospect of natural orifice surgery using flexible endoscopes has led to a renaissance in the practice of surgical endoscopy. Advanced imaging, instrumentation, and techniques facilitate complex endoscopic therapies, which in many cases, supplant traditional surgical management. In order to practice modern gastrointestinal surgery, surgeons must become proficient in standard endoscopy and gain familiarity with the advanced endoscopic options.
Indications and Contraindications for Digestive Endoscopy
Upper endoscopy may be prompted by complaints of dysphagia, odynophagia, or pyrosis. Suspected foreign body impaction, an abnormal barium radiograph, and gastrointestinal bleeding will also indicate a need for endoscopic examination. Additional indications for esophagogastroduodenoscopy include the need for tube gastrostomy for feeding or drainage, persistent vomiting, unremitting epigastric pain, gastric polyposis, and surveillance for neoplasia in patients with conditions predisposing to malignancy such as Barrett’s esophagus, gastric ulcer, pernicious anemia, and previous gastrectomy. In most cases, the modern endoscopist is prepared to deliver therapy during the same procedure during which the diagnosis is established. Therapy may include removal of foreign bodies, dilation of strictures, ablation or stenting of tumors, sclerotherapy or ligation of varices, control of hemorrhage, placement of feeding tubes, and polypectomy.
Colonoscopy is most commonly performed as a screening tool for colorectal cancer. Screening colonoscopy should begin at age 50 in average risk patients. The recommended age for screening is lowered in patients with a family history of colorectal cancer, conditions that predispose to colorectal cancer, and high-risk ethnic groups. Other common indications for colonoscopy include diagnosis of infectious or inflammatory colitis, surveillance for dysplasia or carcinoma in patients with ulcerative colitis, and lower gastrointestinal bleeding. Colonoscopy may be used to decompress dilated bowel in colonic pseudo-obstruction and untwist the sigmoid colon in volvulus.
There are few absolute contraindications for endoscopy. Severe comorbidities and inability to tolerate conscious sedation preclude safe endoscopy. Endoscopic examinations should be performed with caution in patients with recent gastrointestinal anastomoses or inflammation of the gastrointestinal tract, such as diverticulitis. Endoscopy is not without complications and each case should be carefully considered prior to performance of the examination.
Instrumentation
Modern endoscopy is performed with a light source and a video processor attached to a flexible video endoscope. Developments in digital camera technology have allowed the integration of the two modalities so that modern video endoscopes deliver light by a fiberoptic bundle, but transmit the endoscopic image by digital signal to a processor and video screen. The latter allows for a larger, brighter image, which can be easily recorded and electronically enhanced, if necessary.
A large variety of endoscopes are available for diagnostic and specialized therapeutic purposes in adults and children. These include small caliber (9 mm) gastroscopes and pediatric colonoscopes, large caliber (13 mm) and large working channel (6 mm) scopes for complex interventions, mother–daughter scope combinations for biliary and pancreatic ductoscopy, and long slender enteroscopes for small bowel examination. A double-balloon endoscope, which includes an overtube and a scope with a balloon at its tip, enables a large proportion of small bowel to be examined.
There has been a recent expansion of the tools available for therapeutic endoscopy. Biopsy forceps, cytology brushes, and snares are most commonly used for tissue sampling. Rotatable snares and snares of various shapes facilitate difficult polypectomies. Nets may be passed through the working channel for retrieval of large specimens.
Hemostasis may be achieved by contacting the source of bleeding with a probe and applying tamponade and bipolar coagulation at once or by supplying energy without contacting the source, such as argon plasma coagulation. Nonthermal hemostasis can be achieved with hemostatic clips, loops, or bands.
Advanced Imaging in Endoscopy
White light is used for most endoscopic examinations and therapies. Most recent advances in endoscopic imaging enable the early detection of mucosal abnormalities in either the upper or lower gastrointestinal tract. Performing this “optical biopsy” using advanced imaging may direct biopsies and resections to areas of premalignant and malignant change.
In chromoendoscopy, a liquid dye such as methylene blue or Lugol’s solution is applied to the gastrointestinal mucosa. Diseases such as Barrett’s esophagus and squamous cell carcinoma demonstrate differential staining by normal cells and cells with metaplasia or dysplasia.
Narrow band imaging is available on most contemporary endoscopes. By changing the bandwidth of projected light from the endoscope, metaplasia and flat adenomas can be differentiated from normal mucosa.
Optical coherence tomography (OCT) uses reflection of near-infrared light to produce real-time two-dimensional cross-sectional images of the gastrointestinal tract. A small probe, similar to an endoscopic ultrasound probe, is passed through the scope. OCT produces a high-resolution image of the layers of the gastrointestinal tract and might be used for early detection of dysplasia or metaplasia.
Technique of Esophagogastroduodenoscopy
Patients are prepared for upper endoscopy by assuring that their stomach is empty. This usually involves a 4- to 6-hour fast or, in urgent cases, gastric lavage. The patient is positioned on his side with the left side down and sedation is administered intravenously while vital signs and oxygen saturation are monitored. Topical posterior pharyngeal anesthesia may also be used. The endoscope handle is held in the left hand regardless of
the surgeon’s hand dominance. The up and down deflection knob is controlled by the left thumb, while the air, water, and suction buttons are manipulated by the left index and middle fingers. The smaller left–right deflection knob is usually managed by the right hand. The surgeon’s right hand controls the shaft of the endoscope for advancing and torquing the instrument.
the surgeon’s hand dominance. The up and down deflection knob is controlled by the left thumb, while the air, water, and suction buttons are manipulated by the left index and middle fingers. The smaller left–right deflection knob is usually managed by the right hand. The surgeon’s right hand controls the shaft of the endoscope for advancing and torquing the instrument.
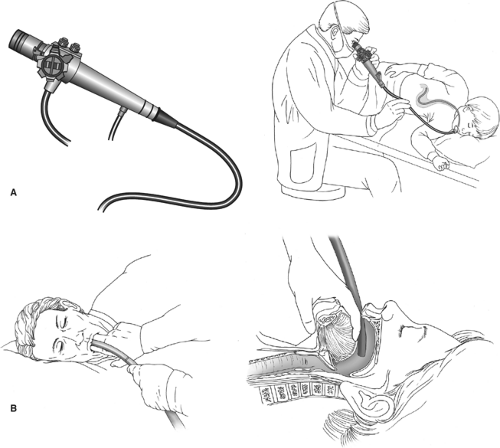
The endoscope may be introduced by digital palpation, the endoscopist’s finger(s) being used to guide the scope, but the safest method of introducing the endoscope into the esophagus is under direct vision (Fig. 1). The endoscope is slowly advanced over the tongue until the epiglottis and vocal cords are visualized. The tip is then angled posteriorly between the arytenoids while the patient is asked to swallow. Gentle pressure is then applied to advance the scope into the esophageal introitus. If the tip of the scope slips into the piriform sinus, it is withdrawn and positioned in the midline above the esophageal introitus, and the patient is asked to swallow again.
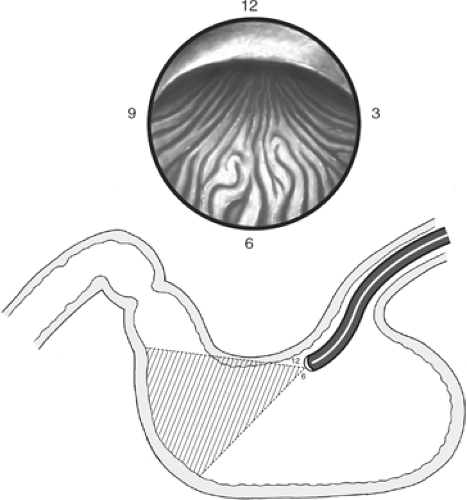
The esophageal lumen appears as a long, straight tube. Once it is entered, air is insufflated. A long view of the esophagus should be obtained and its mucosa inspected. Peristalsis should be noted, and evidence of inflammation or Barrett’s epithelium sought. The endoscope is advanced carefully and only while visualizing the lumen rather than sliding along the mucosa. Interruptions in the normal mucosa by inflammation or suspected neoplasm should be assessed by cytologic brushing and biopsy. A hiatal hernia will appear as a saccular portion of gastric mucosa above the pinching action of the diaphragm. Normally, the esophagus turns slightly to the left as it traverses the diaphragm and enters the abdomen. The esophagogastric junction is noted by the “z” line, an irregular junction of the orange columnar gastric mucosa with the pale pink squamous mucosa of the esophagus (Fig. 2).
The stomach should be fully inflated with air. Any redundancy in the scope should be reduced and the mucosa carefully observed. The scope should be oriented in the gastric body so that the stomach’s posterior wall is at the 3 o’clock position, the lesser curvature at the 12 o’clock position, the anterior wall at the 9 o’clock position, and the greater curvature at the 6 o’clock position (Fig. 3). Small movements of the control knobs will direct the tip of the scope in any desired direction. Abnormalities noted may include ulcerations, gastritis, vascular lesions, neoplasia, or extrinsic impressions upon the gastric wall. The location of such extrinsic impression may indicate the probable source. For example, a large impression on the posterior wall of the stomach, noted at the 3 o’clock position, may indicate a pancreatic mass such as a pseudocyst or tumor.
The endoscope is advanced toward the gastric antrum by following the lesser curvature, and the incisura angularis will be
observed at the 12 o’clock position. It is a smooth curved arch dividing the gastric body from the antrum. Marked elevation of the instrument’s tip and a small turn to the left while the scope is positioned at the gastric angle will usually permit a “retroflexed” view of the gastric cardia and fundus (Fig. 4). The shaft of the scope is then withdrawn to bring the tip of the scope toward the gastric cardia. The shaft of the retroflexed scope is rotated to allow careful assessment of the cardia, fundus, and the lesser curvature.
observed at the 12 o’clock position. It is a smooth curved arch dividing the gastric body from the antrum. Marked elevation of the instrument’s tip and a small turn to the left while the scope is positioned at the gastric angle will usually permit a “retroflexed” view of the gastric cardia and fundus (Fig. 4). The shaft of the scope is then withdrawn to bring the tip of the scope toward the gastric cardia. The shaft of the retroflexed scope is rotated to allow careful assessment of the cardia, fundus, and the lesser curvature.
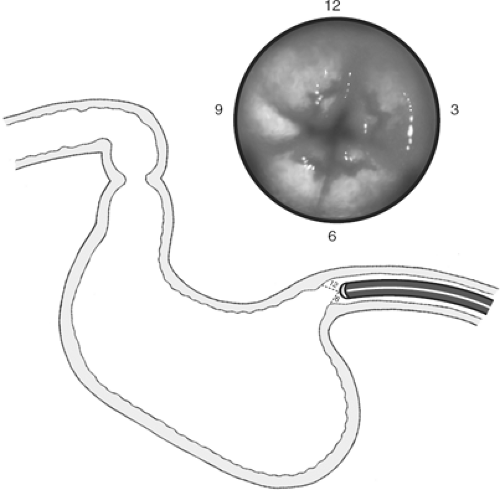 Fig. 2. The “z” line appears at the esophagogastric junction. It represents the change from the squamous esophageal mucosa to the columnar epithelium of the stomach. |
The large rugal folds noted on the greater curvature of the stomach’s body disappear as the antrum is entered. The tip of the scope is elevated to permit its advancement toward the pylorus. Small circular motions of the scope’s tip will permit visualization of the entire antrum. The pylorus is normally round and may be observed to open and close (Fig. 5). Irregularities of the pyloric shape may suggest past or present ulceration. While keeping the pyloric orifice directly in the center of view, the scope is advanced with gentle pressure and slight insufflation of air, into the duodenal bulb. The bulb has no folds, and is a frequent site of inflammation and ulceration. Small motions of the instrument’s tip will help reveal the more obscure corners of the bulb where ulcers may hide (Fig. 6). Once inspection of the anterior and posterior bulb is complete, the scope is advanced into the descending duodenum. This usually requires a maneuver, which is not under direct vision. The scope’s tip is turned to the right as the shaft of the instrument is also rotated to the right. The tip is first moved upward and then down. This turns the instrument posteriorly and then downward into the second portion of the duodenum. Once the lumen of the descending duodenum is in view, slight pressure may be applied to the shaft of the scope to introduce it farther. If resistance is encountered, the scope should be pulled back and straightened. This will usually result in the endoscope advancing further in the duodenum as the redundant gastric loop is straightened. The scope is then withdrawn while close attention is paid to the mucosal detail. The small bowel is noted by the semicircular folds, which are the hallmark of its architecture. The ampulla of Vater may be seen in profile at the 9 o’clock position in the descending duodenum (Fig. 7). Its orifice is usually difficult to observe with the end-viewing panendoscope used for standard upper endoscopy. The accessory papilla, orifice of the duct of Santorini, may sometimes be observed slightly proximal to the ampulla of Vater in the 1 o’clock position. The instrument is pulled back, with the endoscopist slowly manipulating the controls to again survey the walls of the duodenum, stomach, and esophagus.
Therapeutic Interventions During Upper Endoscopy
The ability to deliver endoscopic therapy has increased greatly in recent years as technologies have been developed to permit treatment through the working channel
of the endoscope. These applications have included the ability to coagulate bleeding lesions with monopolar or bipolar probes, or to inject them with long needle injection catheters. Esophageal varices can be treated with injection sclerotherapy or rubber band ligation, controlling hemorrhage and eventually obliterating the large venous trunks. Hydrostatic balloons can be introduced through the working channel to dilate esophageal, anastomotic, and pyloric strictures, and laser energy can be directed through the scope to debulk obstructing esophageal tumors or to treat vascular lesions of the stomach.
of the endoscope. These applications have included the ability to coagulate bleeding lesions with monopolar or bipolar probes, or to inject them with long needle injection catheters. Esophageal varices can be treated with injection sclerotherapy or rubber band ligation, controlling hemorrhage and eventually obliterating the large venous trunks. Hydrostatic balloons can be introduced through the working channel to dilate esophageal, anastomotic, and pyloric strictures, and laser energy can be directed through the scope to debulk obstructing esophageal tumors or to treat vascular lesions of the stomach.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree