TERMS
•Brand name
•Chemical name
•Controlled substance
•Generic name
•Innovator name
•National drug code
•Proprietary name
•Reconstitution
•Trade name
•Unit dose
OBJECTIVES
Upon completion of this chapter, the technician student will be able to:
•Identify brand and generic names on drug labels.
•Identify dosage strengths of medications on drug labels.
•Identify dosage forms and routes of administration on drug labels.
•Identify total volume of drug containers and dosage supply of medications.
•Define and identify NDC numbers and bar codes of drug products.
•Identify directions for reconstitution and preparation of medication products and any other label information.
•Identify lot numbers and expiration dates of drug products.
The importance of reading and understanding labels of all medication forms, preparations, and packaging cannot be emphasized enough. The pharmacy technician is often the person to pick up from storage the product to be dispensed to the patient. Developing the habit of reading the labels three times for verification of right dose, right strength, and right form in addition to expiration date and preparation of the product must begin during this time of technician training.
 Brand and Generic Names
Brand and Generic Names
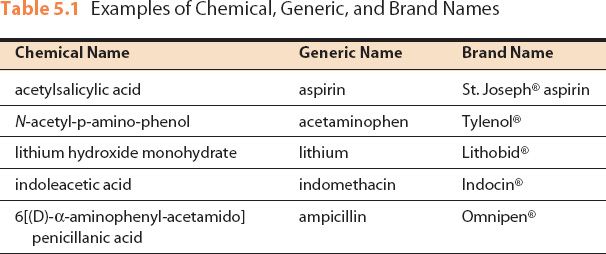
Each drug product actually has three names, the chemical name, the generic name, and the brand name (Table 5.1). The brand name, trade name, proprietary name, and innovator name are all terms used to describe the trademarked name the manufacturer uses. The patent applies to the drug itself; the trademark applies to the brand name the manufacturer uses. There is only one patent on a drug, but after the patent expires, there may be more than one trademarked brand name, such as Valium® and Diastat® for diazepam. The® next to the name indicates that the name is a registered trademark. Also, brand names are usually capitalized. The generic name, which is not capitalized, appears under the brand name on a drug label (Figs. 5.1 and 5.2).
Once the product is not under patent protection and is manufactured as a generic product, often only the generic name appears on the label. Reading the drug label carefully is important because of the many look-alike and sound-alike medications.
Critical Thinking 5.1

Careful reading of the prescription label and the medication or prescription order cannot be emphasized too greatly. For example, Posicor® looks and sounds very similar to Proscar®. When prescriptions for these drugs are handwritten, they can be easily confused. The packaging for these drugs is also similar. Therefore, medication errors have occurred for these look-alike, sound-alike drugs. Another example of look-alike drugs often involved in medication errors are hydralazine, used to control blood pressure, and hydroxyzine, used for allergies or for its sedative effect. Still another example is Plavix®, an antiplatelet drug, being confused with Paxil®, an antidepressant.
The Institute for Safe Medication Practices (www.ismp.org) records errors, helping maintain an awareness not only of look-alike and sound-alike medications but also of medications with similar packaging.
chemical name The name that indicates the chemical makeup of the drug. The generic name is normally some derivative of the chemical name. The chemical name of the drug is normally not a part of the drug label, but it appears on the package insert.
generic name The established name of a drug, usually derived from the chemical name, that appears on the label under the brand name. The generic name is usually in lowercase and is often the name by which the drug is ordered. Once the patent life of a drug expires, other manufacturers are able to manufacture the drug and often use this name.
brand name The name that is under trademark protection from the manufacturer and signified on the label with the symbol®, indicating that the name is registered. Trade name, proprietary name, and innovator name are synonyms of brand name. The brand name of the drug is normally capitalized.
trade name Another term for the brand name, which is under trademark protection from the manufacturer and identified on the label with the symbol®, indicating the name is registered. Proprietary name and innovator name are synonyms.
proprietary name The proprietary name of a drug is another term for the brand name, which is under trademark protection from the manufacturer and indicated on the label with the symbol®, indicating the name is registered. Trade name and innovator name are synonyms. The proprietary name is normally capitalized.
innovator name Another term for the brand name of a drug, which is under trademark protection from the manufacturer and signified on the label with the symbol®, indicating the name is registered. Trade name and proprietary name are synonyms. The innovator name of the drug is normally capitalized.
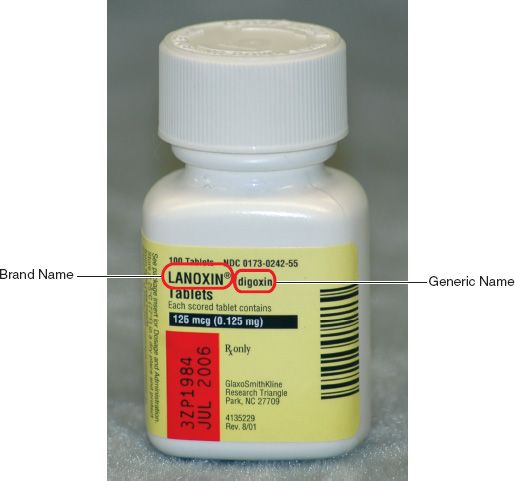
Figure 5.1The brand name of this drug is Lanoxin®; the generic name is digoxin.
Abbreviations to indicate the dosage form are most often immediately to the right of the brand or generic name. For example, the DS in Figure 5.3 indicates double strength. Many initials give us an indication that the drug product was prepared in such a way that its effects last longer than the standard or that the product has delayed dissolving and absorption time. Indications like XR (extended release), CD (controlled dosing), XL (long acting), CR (controlled release), and LA (long acting) are examples of these types of products. Doses for these types of medications are not given as frequently as the standard forms, nor are these products to be broken, crushed, or chewed; therefore, a whole dosage form must be given. The letters EC (enteric coated) indicate that a special coating has been applied to diminish common gastrointestinal effects of the drug. These products also should not be broken, crushed, or chewed.

Figure 5.2This drug does not have a brand name. Its generic name is codeine.
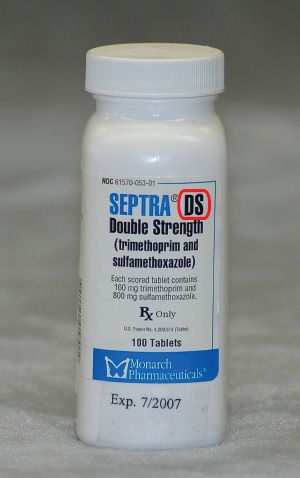
Figure 5.3Abbreviations are used to indicate the dosage form. For example, the DS in the brand name Septra® DS denotes double strength.
 Dosage Strength
Dosage Strength
The dosage strength indicates the amount of drug in a particular dosage form, for example, the weight of drug in a tablet or capsule. The fluconazole label (Fig. 5.4) indicates that each tablet contains 200 mg of active ingredient. This does not mean that each tablet weighs 200 mg. The label on the penicillin VK suspension (Fig. 5.5) shows that each 5 mL of suspension contains 125 mg of penicillin. The epinephrine injection label (Fig. 5.6) indicates a dosage supply of 0.1 mg/mL, and the total volume of the ampul is 10 mL.

Figure 5.4The dosage strength of this dosage form of Diflucan® (fluconazole) is 200 mg.

Figure 5.5The dosage strength of this drug is 125 mg (200,000 units) penicillin V in 5 mL.

Figure 5.6The dosage strength of this injection is 0.1 mg/mL.
 Dosage Form
Dosage Form
Every drug label indicates the form of the drug, whether tablets (Fig. 5.7), capsules, elixir (Fig. 5.8), oral suspension, otic suspension, ophthalmic suspension, powder for suspension, powder for inhalation (Fig. 5.9), topical application, or other form.

Figure 5.7The dosage form of this drug is tablets.
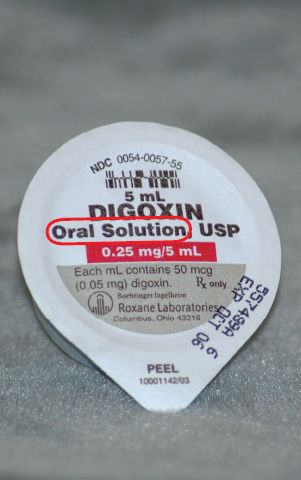
Figure 5.8The dosage form of this drug is elixir, or oral solution.

Figure 5.9The dosage form of this drug is inhalation powder.
 Total Volume
Total Volume
The total volume of the container is indicated in addition to the dosage supply. The volume of the container in the case of oral solid dosage forms like tablets and capsules is simply the total number of units in the container (Fig. 5.10). The volume of liquid medication is the indicated liquid volume, normally measured in milliliters. Total volume of topical medications like ointments, creams (Fig. 5.11), and many inhalation products are indicated by weight and measured in grams.
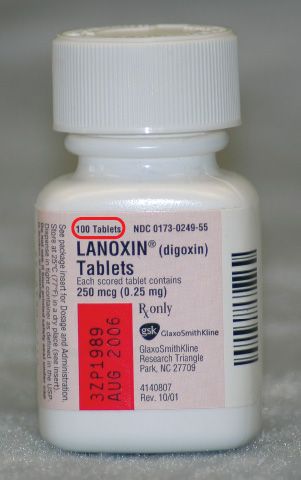
Figure 5.10For solid dosage forms, the volume of the container is the total number of units the container holds. For example, this bottle of Lanoxin holds 100 tablets.

Figure 5.11For topical medications, total volume is indicated by weight.
 National Drug Code
National Drug Code
National drug code (NDC) numbers are assigned to every drug product, both legend (prescription) and OTC (over-the-counter). This code is a specific sequence of numbers, grouped in three sets, used to identify every drug product. The first set of numbers identifies the manufacturer; every drug manufactured by a particular company has the first group of numbers. The second set of numbers identifies the drug itself. All McNeil Tylenol #3® products, for example, have the same set of numbers for the first two groups of numbers: the first for the manufacturer, McNeil, and the second for Tylenol #3®. The last set indicates the package size the product was dispensed from or the package size purchased by the patient in the case of OTC products.
national drug code An identification number that is unique to each drug product. Federal law requires an NDC for every drug product. It consists of three sets of numbers: the first indicates the manufacturer; the second, the name and strength of the drug; and the third, the package size of the drug.
The pharmacy technician uses these numbers for cross-checking prescription labels with the labels of the stock product to avoid medication errors. The pharmacy technician also uses these numbers when filing third-party, Medicare, and Medicaid prescription drug claims. NDC numbers are extremely valuable for insurance claims, cross-referencing drug products, and checking prescription orders against filled prescriptions. Figure 5.12 shows an NDC number on a drug container.

Figure 5.12Every drug product has a unique NDC number. The NDC number of diazepam is circled.
 Bar Codes
Bar Codes
Bar codes (Fig. 5.13) are used by controllers, purchasing agents, and retailers for pricing and ordering. The bar code generally has the NDC number next to it; this is important in the case of OTC medications, as sometimes this is the only place the NDC number appears.

Figure 5.13Each drug bottle has a bar code.
 Lot Numbers and Expiration Dates
Lot Numbers and Expiration Dates
Federal law requires that all medication packages be identified with a lot number or control number. Lot numbers are used to track products in the case of mandatory or voluntary recalls from the Food and Drug Administration (FDA) or the manufacturer. This allows the product to be removed from the shelves and warehouses in a quick, efficient manner. In the recent past, these lot numbers or control numbers have been used to recall OTC products, vaccines, and various prescription drug products.
Expiration dates appear on all manufactured medication packages to notify the pharmacy, distributor, and patient when the product is too old to be used. Some products lose potency; others actually degenerate into a toxic substance. The pharmacy technician must take note of all expiration dates prior to filling a medication or prescription order. Products on pharmacy shelves are checked regularly, removed from stock, and returned to the distributor when they have reached their expiration date. Other products must be marked with an expiration date when prepared for patient use, such as oral suspensions and some injectable and intravenous products. The pharmacy technician must note the storage and expiration dates provided on the medication label, noting the time and date the product must be discarded and how it should now be stored.
The lot number and expiration date of a particular drug bottle are circled in Figure 5.14.

Figure 5.14A lot number and expiration date must appear on all drug products.
 USP/NF
USP/NF
Very often the initials USP appear next to the name of the drug on the label (Fig. 5.15). USP indicates United States Pharmacopoeia, one of two national formularies, or national listing for drug products. NF indicates National Formulary.
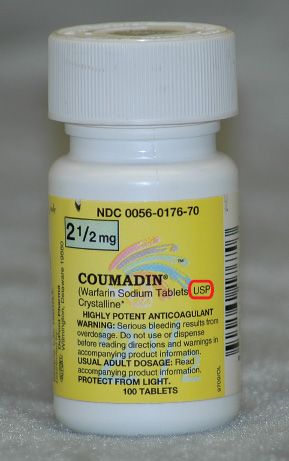
Figure 5.15USP on a drug label indicates that the drug was formulated according to the standards of the United States Pharmacopeia.
 Additional Label Information
Additional Label Information
Other information important to the pharmacy technician contained on medication labels includes directions for reconstitution (Fig. 5.16). Many injectable products contain information for mixing the products for different dosage supplies; sometimes this information is contained on the package insert. This information also includes the type of product or diluent that must be used for reconstitution.

Figure 5.16This penicillin VK label includes directions for reconstitution.
reconstitution Adding a diluent, such as purified water, bacteriostatic water, or other liquid as per package directions, to a powder drug product to make a solution or suspension. Constitution is another word for this process.
Storage information (Fig. 5.17) is another important piece of information for the pharmacy technician. Storage is extremely important. Proper storage temperature—room temperature, refrigerator, or a hot box, as in the case of mannitol—and lighting are important. If medication products are not stored properly, the potency and expiration time of the product may be affected.

Figure 5.17This Dyazide® label contains storage information.
If a drug is a controlled substance, that fact is indicated on the label with a C and the Roman numeral indicating the drug’s schedule category (for example, CII, CIII, CIV, or CV). These symbols appear on the drug label in a number of versions—sometimes as a watermark and other times next to the drug name (Fig. 5.18). Controlled drugs are assigned a schedule on the basis of abuse potential, with a CII drug having a high potential for abuse leading to severe psychological or physical dependence and a CV having the lowest abuse potential. (CI drugs, such as heroin and LSD, are not legal in the United States.)

Figure 5.18The label of a controlled substance includes a special symbol that shows its schedule category.
controlled substance A drug or chemical defined by the Comprehensive Drug Abuse Prevention and Control Act of 1970 to have a potential for abuse. These drugs are ranked in five categories ranging from those with a high potential for abuse to those with little potential for abuse. Drugs in the first category are not legally sold or used in the United States and have the highest potential for abuse. Controlled substances are marked with a C and a roman numeral indicating which category they belong to.
The manufacturer’s name also appears on every medication label. Manufacturers that are subsidiaries of a larger company will be noted on the medication label as well. Often the address of the manufacturer is also present. Figures 5.19 and 5.20 show examples.
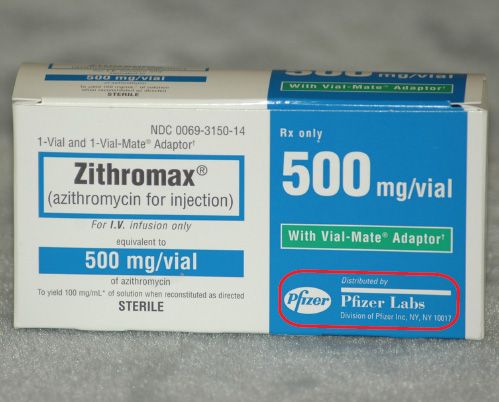
Figure 5.19The manufacturer of Zithromax® is Pfizer Labs, a division of Pfizer, Inc., in New York City.
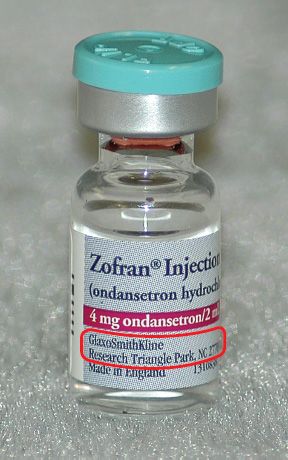
Figure 5.20The manufacturer of Zofran® is GlaxoSmithKline, in Research Triangle Park, North Carolina.
 Combination Drug Labels
Combination Drug Labels
Many drug products are combinations of two or more drugs in a single dosage form. The labels of these medications include the name and quantity of each component drug. The medication order or prescription for these drugs indicates the number of tablets, capsules, or milliliters to be used for one dose, normally not the strength of each drug. Figures 5.21 and 5.22 show examples of combination drugs.
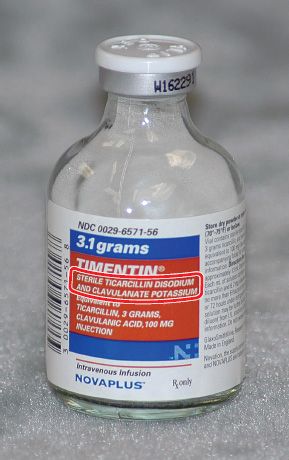
Figure 5.21Timentin® is a combination drug. The two ingredients that make up this drug are circled.

Figure 5.22Dyazide® is a combination drug. The two ingredients that make up this drug are circled.
 Unit Dose Labels
Unit Dose Labels
Oral medications used in hospital settings are most often packaged in a unit dose, or single dose (Figs. 5.23 and 5.24). This type of packaging requires all of the same information as for other types of labeling. The only item missing is the total volume, since the contents of the package is understood to be one dose. Many of these products are commercially available in blister packs or strip packaging. Many hospitals package their own unit-dose medications, using robots or packaging machines.

Figure 5.23The front (top) and back (bottom) of a unit dose of phenobarbital are shown here.
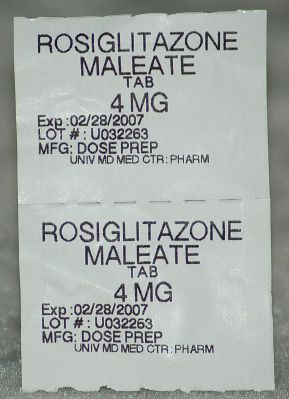
Figure 5.24Two unit doses of rosiglitazone.
unit dose Single dose units of tablet, capsule, or liquid medication labeled the same as bulk medication with the exception of total volume. The dosage strength is understood as per one. Many manufacturers supply institutions with unit dose medications; when a drug is not available in a unit dose package, institutions package their own.
Rules for Understanding Drug Labels