TERMS
•Enlarging
•Proportional parts
•Reducing
•USP/NF
OBJECTIVES
Upon completion of this chapter, the technician student will be able to:
•Determine the correct quantities of individual ingredients required to enlarge or reduce any given formula or recipe for pharmaceutical compounds using quantities by weight or volume.
•Determine the correct quantities of individual ingredients required to enlarge or reduce any given formula or recipe for pharmaceutical compounds using quantities expressed in parts.
Pharmacists or technicians may have to reduce or enlarge formulas for pharmaceutical preparations in the course of their professional practice or manufacturing activities. Official (USP/NF) formulas generally are based on the preparation of 1000 mL or 1000 g. Other formulas, such as those found in the literature, may be based on the preparation of a dosage unit (e.g., 5 mL, 1 capsule) or another quantity (e.g., 100 mL). Industrial formulas may be scaled up to quantities of ingredients sufficient to prepare hundreds of thousands of dosage units in a production batch. It is common for pharmacies to keep a recipe box with formulas that they commonly compound. These recipes are adjusted to make individual prescription quantities according to the medication or prescription order received. In each of these instances, a pharmacist or technician may calculate the quantities of each ingredient required for a smaller or greater quantity by reducing or enlarging the specified formula while maintaining the correct proportion of one ingredient to the other. This is the same process used to double or halve a kitchen recipe or mix gas and oil.
USP/NF The abbreviation for United States Pharmacopeia/National Formulary.
reducing Calculating the amounts to be used in a pharmaceutical formula to make a smaller amount than the original formula.
enlarging Calculating the proper amounts to be used in a pharmaceutical formula to make a larger amount than the original formula.
Example:
A recipe makes 6 dozen rolled sugar cookies. You are asked to bring 3 dozen cookies to your child’s classroom to be decorated at the class party and 5 dozen to a cookie exchange. You also want to freeze some for the holidays. Therefore, you want to make 12 dozen cookies. You are doubling the quantity so you must multiply each ingredient by 2. The ratio is 1:2. The recipe is as follows:
| 1 cup shortening | 3 tsp baking powder |
| 2 cups sugar | 1 tsp vanilla |
| 6 eggs | 5 cups flour |
To find the amount of shortening in the doubled recipe:
![]()
X = cups shortening, answer
Or simply multiply each ingredient by 2 to get the proper amounts to prepare 12 dozen cookies.
The skills learned in this chapter will be put into practice during compounding laboratory experience. Therefore, when a formula specifies a total quantity (e.g., 1000 mL), it may be necessary to determine how much of each ingredient is needed to prepare a different total quantity (e.g., 60 mL) by this equation:

X = Quantity of an ingredient for the quantity of formula desired.
If a formula for 1000 mL contains 6 g of ingredient A, how much of this ingredient (in grams) is needed to prepare 60 mL of the formula?
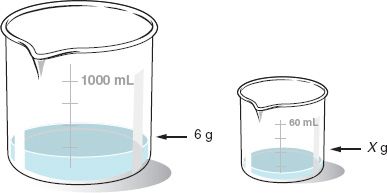
![]()
X = 0.36 g ingredient A, answer
Although all problems specifying a total amount may be solved by this equation, it is often more convenient to use shortcuts in calculations. For example, to reduce or enlarge a metric quantity by a power of 10, the decimal point may be moved to the left or right the required number of places. That is, if a formula for 1000 mL is to be reduced to 100 mL, each component in the formula also may be reduced by a factor of 10 by moving the decimal point one place to the left to make only 10% of the original formula. For example, if 39 g of an ingredient is called for in a formula to prepare 1000 mL, then 3.9 g, or 10% of it, would be used to prepare 100 mL of the formula. To prepare 1 gal (3785 mL) of the formula, multiply the quantity of each ingredient in the formula by the factor 3.785, the multiple of the formula to prepare (3785 ÷ 1000 = 3.785). Or to prepare 50 mL, or ![]() or 5%, of the formula, multiply each ingredient by
or 5%, of the formula, multiply each ingredient by ![]() or by 0.05 (5%).
or by 0.05 (5%).
Some formulas, however, do not specify a total amount, instead indicating relative quantities of ingredients, or proportional parts, to be used in obtaining any desired total amount. For example:
proportional parts The relative quantities of ingredients in any given formula.
| Salicylic acid | 1 part |
| Precipitated sulfur | 8 parts |
| Hydrophilic ointment | 91 parts |
To determine the amounts of each ingredient to be used in a specified amount of the formula, the following equation may be used:

From this formula, calculate the quantity of salicylic acid to use in preparing 30 g of ointment.
![]()
X = 0.3 g, salicylic acid, answer
Rules for Reducing and Enlarging Formulas

•To make a valid ratio, the total amounts compared (i.e., that specified and that desired) must be expressed in a common denomination, whether in grams, fluidounces, milliliters, or another unit. If they are unlike from the start, one or the other must be reduced or converted. In general, it is best to convert units in other systems to metric units.
•Because the quantity of each ingredient is calculated separately when reducing or enlarging a formula, it does not matter if the individual ingredients are of different units (e.g., grams, milliliters, fluidounces).
 Formulas That Specify Amounts of Ingredients
Formulas That Specify Amounts of Ingredients
Calculating Quantities of Ingredients When Reducing or Enlarging a Formula
Calculating the quantities of ingredients to be used when reducing or enlarging a formula for a specified total amount involves the following.
Examples:
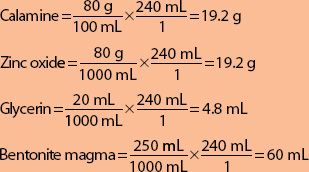
From the following formula, calculate the quantity of each ingredient required to make 240 mL of calamine lotion.
| Calamine | 80 g |
| Zinc oxide | 80 g |
| Glycerin | 20 mL |
| Bentonite magma | 250 mL |
| Calcium hydroxide topical solution, to make | 1000 mL |
Use the factor 0.24 (because 240 mL is 24% of 1000 mL) to make only 24% of the original quantity. The quantity of each ingredient is calculated as follows:
Calamine = 80 × 0.24 = 19.2 g
Zinc oxide = 80 × 0.24 = 19.2 g
Glycerin = 20 × 0.24 = 4.8 mL
Bentonite magma = 250 × 0.24 = 60 mL
Calcium hydroxide topical solution, to make 240 mL, answers
Solve by Dimensional Analysis
Calcium hydroxide topical solution, tomake 240 mL, answers.
To make 60 mL, move the decimal point one place to the left and multiply by 0.6. This will produce 60% of the original quantity. To make 50 mL, divide by 20 or multiply by 0.05 (5%) or move the decimal point one place to the left and divide by 2. To make 125 mL, multiply by one-eighth or 0.125 (because 125 mL is one-eighth or 12.5% of 1000 mL). Arrive at these percents by dividing the quantity to make by the quantity of the original recipe.
Critical Thinking 8.1

Remember, multiplying a quantity by a factor does not change the unit of measure. It simply increases or decreases the number of units. So if the quantity is to be in grams of each ingredient and the recipe is in milligrams, the result is in milligrams after multiplication, and the quantity must be converted to grams.
From the following formula, calculate the quantity of each ingredient required to make 1 gal of compound benzoin tincture.
| Benzoin | 100 g |
| Aloe | 20 g |
| Storax | 80 g |
| Tolu balsam | 40 g |
| Alcohol, to make | 1000 mL |
Use the factor 3.785 to make 3.785 times as much as the original recipe. (Divide 3785 by 1000—the quantity to make divided by the formula quantity.) The quantity of each ingredient is calculated as follows:
Benzoin = 100 × 3.785 = 378.5 g
Aloe = 20 × 3.785 = 75.7 g
Storax = 80 × 3.785 = 302.8 g
Tolu balsam = 40 × 3.785 = 151.4 g
Alcohol to make 3785 mL or 1 gal, answers
From the following formula, calculate the quantity of each ingredient required to make 1 lb of the ointment.
| Coal tar | 50 g |
| Starch | 250 g |
| Zinc oxide | 150 g |
| Petrolatum | 550 g |
The formula is for 1000 g. The factor is 0.454 (divide the quantity to make by the original quantity, or 454 g ÷ 1000 g). The quantity of each ingredient is calculated as follows:
Coal tar = 50 × 0.454 = 22.7 g
Starch = 250 × 0.454 = 113.5 g
Zinc oxide = 150 × 0.454 = 68.1 g
Petrolatum = 550 × 0.454 = 249.7 g
454 g or 1 lb, answers
Rule for Adding Weights




