Fig. 31.1 Muscle anatomy of the thoracic outlet.
Structures of interest contained within or traversing the region of the thoracic outlet include;
- bone: clavicle, first rib, C7 vertebra, cervical rib (if present), fibrous extensions from C7 (if present)
- muscles: anterior, middle scalenes, subclavius, pectoralis minor tendon
- nerves: brachial plexus including the trunks (supraclavicular), divisions (retroclavicluar) and cords (infraclavicular); axillary and musculocutaneous nerves (infraclavicular)
- vessels: subclavian artery and vein, axillary artery and vein.
Sites of entrapment of nerves
Reported sites of entrapment of nerves or vessels include compression between:
- anterior and middles scalene muscles: neurogenic symptoms in the distribution of T1 to C8 nerve roots. Compression is attributed to an enlarged anterior or middle scalene, muscle spasm or a fibrous band within the anterior scalene
- anterior scalene and cervical rib or anterior scalene and a fibrous band extending from C7: symptoms may be neurogenic or arterial/vasogenic, including decreased pulse, cool, pale extremity or Raynaud’s symptoms
- clavicle and first rib: largely post-traumatic (most often at the level of the cords of the brachial plexus or axillary vessels) following a mid-shaft clavicle fracture with robust callus formation; the callus may compress neurovascular elements and patients most often present with combined neurovascular TOS symptoms
- beneath the pectoralis minor muscle or tendon: compression may be neurogenic (medial or lateral cord), arterial (axillary) or venous (axillary).
Epidemiology
Thoracic outlet syndrome is a rare disorder. The reported incidence of true neurogenic TOS approaches 1 in a million. Given that neurogenic TOS is diagnosed in 95% of those with TOS, the vasogenic causes will be exceedingly rare (Torriani et al., 2010). Given the vague symptoms and lack of anatomical correlates, there can be no good information about non-specific TOS.
Work up and differential diagnosis
Neurogenic, arterial/vasogenic and veno-vasogenic TOS have objective criteria and can be evaluated with clinical, electrophysiological and vascular studies. When a patient presents with pain in the arm with or without numbness, the first thought should be cervical spine disease even if anatomical findings are not obvious. It is when anatomical findings are not obvious that the diagnosis of non-specific TOS is often considered (Ferrante, 2012; Foley et al., 2012). This diagnosis should be made only rarely, hopefully when at least some objective evidence is documented.
Clearly establishing a diagnosis should be the first step when evaluating a patient with suspected TOS. Given the various presentations and perplexing symptoms associated with TOS, this often requires extensive diagnostic testing including imaging, electrodiagnostic testing and/or vascular studies. A full discussion of this topic is beyond the scope of this chapter and the reader is referred to several reviews (Urschel and Kourlis, 2007; Ferrante, 2012; Foley et al., 2012).
Diagnostic testing is directed by the patient’s history, symptoms. Electrodiagnostic testing including nerve conduction and EMG should be considered in patients presenting with neurogenic TOS symptoms, and such testing should be definitive. While a normal electrodiagnostic test result does not exclude other forms of TOS, the test may also reveal an alternative cause of the patient’s symptoms such as other peripheral nerve entrapments, peripheral neuropathy, plexopathy, radiculopathy or focal anterior horn cell loss (Tsao, 2007; Urschel and Kourlis, 2007; Ferrante, 2012). Abnormal results for the median, ulnar or medial antebrachial cutaneous sensory nerve action potentials have been reported in TOS, and abnormality of the ulnar potentials from the fifth finger is almost mandatory for diagnosis of neurogenic TOS. Denervation in those muscles innervated by C8–T1 is also characteristic, and atrophy in these muscles may also be seen in long-standing neurogenic TOS (Ferrante, 2012; Foley et al., 2012).
Diagnostic imaging in patients with TOS generally includes plain radiographs to evaluate for a cervical rib, clavicular callus, other bony abnormalities or mass effect. Fibrous bands will not be visible on plain films but are detected by MRI. Imaging with MRI or CT may also be useful when an apical lung mass, tumor or malignancy is suspected (Ferrante, 2012).
When venovascular symptoms predominate, venography is the gold standard. For patients with suspected arterial vasogenic TOS, MRI, CT angiography or duplex ultrasonography should be considered (Stapleton et al., 2009). High-frequency ultrasound is useful for both procedural guidance and as a diagnostic tool for arterial vasogenic TOS and has the advantage of portability, lower cost and no exposure to ionizing radiation. Ultrasound in B-mode can also provide a dynamic assessment of vascular flow and can be used during provocative clinical maneuvers to assess the effect of a maneuver or neck/limb position on flow within a vessel (Jordan et al., 2007; Danielson and Odderson, 2008; Odderson et al., 2009; Torriani et al., 2010). It is important to recognize that arterial or venous compression with various maneuvers is also seen in asymptomatic individuals, so thoughtful clinical correlation is needed.
Treatment
Various treatments for TOS have been proposed including postural or physical therapy, manipulation, acupuncture, BoNT injections and various surgical procedures. The focus of this text is on BoNT therapy for diagnostic or therapeutic intervention for TOS. There are several excellent reviews that contain a full discussion of the various proposed treatments for TOS (Jordan et al., 2007; Ferrante, 2012; Foley et al., 2012). Only certain therapies make sense for certain conditions. For example, if the brachial plexus is compressed by a first rib, there is no way in which BoNT can help and surgery would be necessary. The utility of BoNT is currently supported only by anecdotal case reports, and there is certainly no logic in using BoNT therapy if entrapment by a muscle is not the issue.
Botulinum neurotoxin therapy under ultrasound guidance
As noted above, BoNT therapy for TOS is associated with risks related to the needle insertion/injection procedure itself as well as the risks inherent with BoNT. It is incumbent on the clinician to establish both a diagnosis of true TOS and the correct site of compression and the involved structures prior to proceeding with BoNT therapy. Clearly, BoNT has no place in treatment of TOS caused by callus formation from a clavicle fracture or compression from a true cervical rib. Additionally, BoNT has no place in treatment of non-specific TOS where no objective compression can be identified. However, BoNT may be useful for patients with compression of neurovascular structures caused by the effects of muscle hypertrophy or muscle action on bones (anterior scalene on first rib), leading to TOS.
Since the mid 2000s, several reviews of ultrasound-guided BoNT therapy have been published (Jordan et al., 2007; Danielson and Odderson, 2008; Le et al., 2010; Torriani et al., 2010; Foley et al., 2012).
Techniques
Ultrasound guidance for TOS has been described for BoNT injections in the anterior and middle scalene, pectoralis minor and subclavius muscles. Accurately localizing any of these muscles may be challenging without imaging-based guidance, such as ultrasound or fluoroscopy.
A general review of ultrasound and ultrasound guidance techniques useful for BoNT injections is provided elsewhere in this text (e.g. Chapter 7). For a more detailed review of ultrasound guidance and procedural techniques, see Smith and Finnoff (2009), Alter (2010) and Alter et al. (2012).
Both the in-plane and out-of-plane injection techniques are useful when performing ultrasound-guided BoNT injections for TOS. Clinicians should be at ease with both techniques and familiar with the advantages and disadvantages of both. Therefore, a brief discussion, specific to the topic of these techniques for TOS is provided below (Smith and Finnoff, 2009; Alter, 2010). Because of anatomical variations, patient-related issues with positioning and other factors, one specific technique may be required for a given procedure.
In an out-of-plane injection technique, the needle is inserted across the short axis of the transducer (Fig. 31.2). Using this technique, a cross-sectional view of the needle is obtained and the needle is viewed as a bright (i.e. hyperechoic) dot (Fig. 31.3). The out-of-plane technique may provide a more direct path to the target. However, the entire length of the needle and its path is not visualized. During needle insertion, care must be used to keep the tip of the needle within the ultrasound beam. If the tip is inserted beyond the ultrasound transducer, it may be in an untargeted structure. When using the out-of-plane technique to track the path of the needle to the target, a walk-down technique is recommended. In this approach, the needle is vibrated or jiggled during insertion. Jiggling the needle creates movement in the tissue through which the needle is passing. The clinician tracks the position of the needle by observing movement of tissue as the needle passes from superficial through deeper structures. When the needle tip reaches the target, a small quantity of the injectate is injected, confirming the correct location.
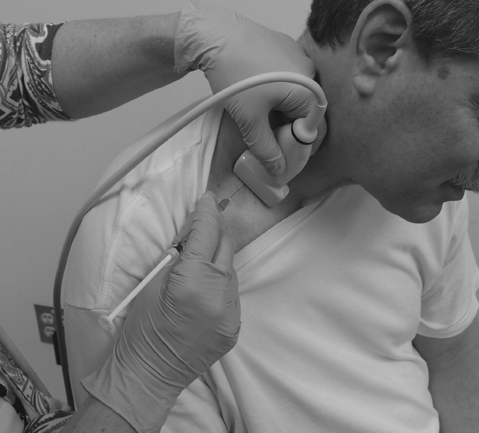
Fig. 31.2 Needle/transducer orientation for transverse ultrasound out-of-plane injection of the scalene muscles.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


