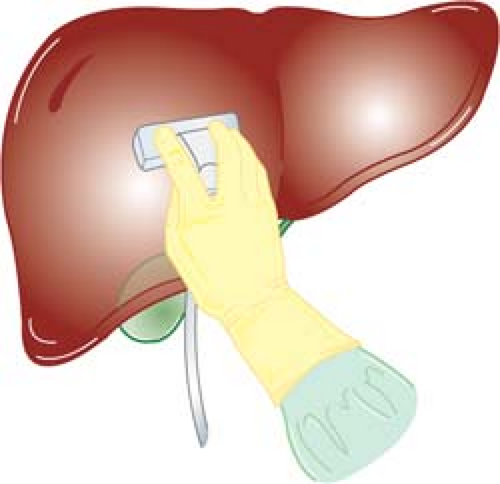
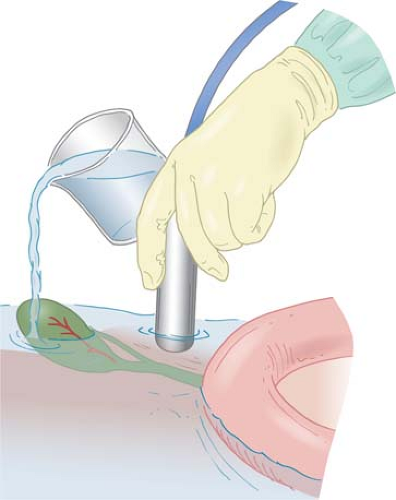
Ultrasonography by Surgeons
Junji Machi
Introduction
Ultrasound is the only imaging modality that can be used by surgeons widely during various types of operations. It provides valuable intraoperative information once appropriately performed by surgeons. In spite of advances of various imaging techniques employed preoperatively, surgeons often need to make final decisions on surgical indications or type of operations to be performed during surgery based on intraoperative findings. Meticulous exploration by inspection and palpation may not always be accurate or possible; this is particularly true in evaluation of solid organs or deep structures. Intraoperative imaging methods can provide useful and, at times, critical information. Intraoperative ultrasound (IOUS) is a valuable and widely applicable modality because it provides high-resolution images of the operative field in real time, which cannot be obtained by other intraoperative studies.
While a laparoscopic approach has a number of advantages, it has certain limitations, in particular, the lack of sufficient tactile feedback from the tissues and little or no capability of palpation. Laparoscopic ultrasound (LUS) is a form of IOUS, which represents a recent merger in the laparoscopic and IOUS technologies, and allows surgeons to visualize and evaluate the interior of organs and deep structures. This imaging capability of LUS can remarkably compensate for the inherent limitation of laparoscopic examination.
It is 30 years since high-resolution real-time B-mode ultrasound was first introduced in the operating theater as IOUS. The value of this imaging tool has since been demonstrated in a variety of surgical fields including general surgery, neurosurgery, cardiovascular surgery, urology, and gynecology. With increasing number of laparoscopic operations being performed, the application of LUS has also been expanding. I have performed IOUS/LUS in >5,000 operations, and have identified indications, benefits, and limitations. My recent major application of IOUS/LUS has been in hepatobiliary pancreatic surgery. In this chapter, I review the indications, advantages, and limitations of IOUS and LUS, in addition to technical issues. Endoscopic and endorectal ultrasound, advances, and perspective of surgeon’s ultrasound are also discussed.
There are four general indications of IOUS during open surgery: (a) acquisition of new diagnostic information not otherwise available, (b) complement to or replacement for conventional operative radiography, (c) confirmation of completion of operation, and (d) guidance of surgical procedures.
Acquisition of new information that is not obtained by preoperative studies or intraoperative exploration includes new diagnosis of diseases, localization, or exclusion of previously suspected lesions, and acquisition of other anatomic information. IOUS helps to assess the extent of malignant disease (tumor staging). For example, vascular invasion and lymph node and liver metastasis of hepatobiliary, pancreatic, and gastrointestinal carcinoma can be diagnosed more accurately than by preoperative methods. IOUS has changed previously planned surgical procedures for hepatic tumors in 30% to 50% of operations. When used as a screening procedure, IOUS has detected occult liver metastases from colorectal carcinoma in 5% to 10% of operations. Precise localization of nonpalpable lesions such as hepatic tumors, liver cysts and abscesses, intrahepatic calculi, pancreatic cysts, and dilated pancreatic ducts is possible. For example, as many as 40% of hepatocellular carcinomas in cirrhotic liver are not palpable during operation, but can be readily localized by IOUS. IOUS is now regarded as one of the best means to localize islet cell tumors. In addition to diagnosing focal lesions, important anatomic structures (e.g., vascular structures, bile ducts) can be
identified prior to extensive dissection. By providing new imaging information early during operation, IOUS facilitates intraoperative decision making and the selection of the most appropriate surgical operation.
identified prior to extensive dissection. By providing new imaging information early during operation, IOUS facilitates intraoperative decision making and the selection of the most appropriate surgical operation.
IOUS is useful as a complement to or replacement for operative radiographic studies. In comparison to operative cholangiography during open cholecystectomy (studies in 1980s to 1990s), IOUS has demonstrated equal or superior accuracy in diagnosing bile duct calculi. We believe that IOUS can replace traditional operative cholangiography as a routine screening test. Currently, while most of the cholecystectomies are performed by laparoscope, open biliary surgery is used for more complicated biliary problems. IOUS can be valuable not only for diagnosing bile duct calculi, but also for clarification of biliary anatomy.
Confirmation of completion of operation includes both the assessment of adequate tissue resection or calculus and foreign body extraction and discovery of technical problems. For confirmation of completion of operation, IOUS can be used after removal of biliary or renal calculi, extraction of foreign bodies and extirpation of tumors in solid organs (e.g., liver, pancreas). During peripheral vascular surgery, IOUS has shown equal accuracy to operative arteriography in detecting vascular defects, such as intimal flaps, strictures, and thrombi. Therefore, IOUS can be used as a completion examination immediately after vascular reconstructions. More recently, completion IOUS has become important during organ transplantation. After liver, pancreas, or kidney transplantation, color/power Doppler imaging can be used to evaluate the vascular anastomosis and to assess adequate blood flow to organs intraoperatively.
Ultrasound is an ideal modality to guide surgical procedures in the operating room. Ultrasound guidance of various manipulations during operation has the advantage of real-time imaging without using ionizing radiation. The detail of IOUS-/LUS-guided procedures is discussed in the following section.
In general, the indications of LUS are essentially similar to those of IOUS during laparotomy. Among various indications, there are two major situations in which LUS is frequently performed: (a) examination of the bile duct during laparoscopic cholecystectomy and (b) staging and determination of resectability during laparoscopic exploration for abdominal malignancies.
More than 10 prospective studies have compared LUS and operative cholangiography during laparoscopic cholecystectomy during 1990s. The time required to complete LUS ranged from 4 to 10 minutes, with an average of <7 minutes, about half of the time needed for operative cholangiography. The success rate in completing the examination was comparable for LUS and operative cholangiography. The results of LUS and operative cholangiography in diagnosing bile duct calculi were comparable except for better LUS in terms of the positive predictive value and specificity. More recent studies have shown that LUS can delineate biliary anatomy clearly, and the routine use of LUS might help to significantly reduce the need for cholangiography and moreover to decrease bile duct injuries. LUS cannot entirely replace operative cholangiography, and both should be used in a complementary fashion. However, LUS can become the first-choice procedure during laparoscopic biliary surgery because of its safety, speed, and cost-effectiveness.
LUS is indicated whenever laparoscopic exploration is performed for abdominal malignant tumors. Laparoscopy has been shown to more correctly predict the resectability of abdominal tumors than preoperative studies. Laparoscopic inspection is effective to detect peritoneal dissemination and metastasis to the liver surface, but not to evaluate structures or organs under the surface. LUS delineates tumors deep in the parenchyma of organs, especially solid organs such as the liver. The retroperitoneal structures are visualized without tissue dissection. First, local tumor invasion to other structures, particularly blood vessels can be evaluated by LUS. Second, liver and lymph node metastases are assessed in a manner similar to IOUS at open surgery. Studies of malignant liver tumors showed that LUS demonstrated liver tumors that had been imperceptible to laparoscopic inspection in 20% to 40% of patients. Further information regarding the resectability, such as major vascular invasion or lymph node metastasis, was obtained by LUS in 10% to 30%. Similar results were reported by studies of laparoscopy with LUS on pancreatic cancer, biliary cancer, and gastroesophageal cancer. Laparoscopic exploration prior to planned laparotomy will be increasingly utilized for various abdominal malignancies, and LUS is capable of improving the staging accuracy, thereby decreasing unnecessary or nontherapeutic laparotomies.
A number of other applications have been suggested by recent reports on LUS during laparoscopic surgery or exploration. These include evaluation and biopsy guidance of uncertain liver lesions or liver tumors of unknown pathology, guidance of laparoscopic ablation of liver tumors, assistance during laparoscopic surgery of liver cysts, guidance of laparoscopic liver resection, evaluation of gallbladder polyps, detection and drainage assistance of pancreatic pseudocysts, localization of pancreatic islet cell tumors, guidance of pancreatic resection (particularly distal pancreatectomy), assistance during laparoscopic adrenalectomy, evaluation of splenic lesions and localization of accessory spleens, evaluation and biopsy guidance of other intraperitoneal and retroperitoneal tumors or lymph nodes, assistance of drainage of abscesses or fluid collections, and so forth. Particularly during laparoscopic colorectal surgery, which is becoming a standard treatment, LUS will be a valuable tool to evaluate the liver and lymph nodes for metastases. As laparoscopic surgery continues to apply to the larger numbers and various types of abdominal diseases, the application and utility of LUS during laparoscopic surgery are expected to increase.
Advantages and Limitations of Intraoperative Ultrasound
Compared to preoperative studies and operative radiography, IOUS has a number of advantages including safety, repeatability, speed, high accuracy, more imaging information, wider applicability, and procedure guidance capability. IOUS is inherently safer, and therefore can be used repeatedly as necessary during the course of operation. IOUS can be performed in a short period of time. For example, biliary and liver IOUS for the purpose of screening can be completed within 5 minutes. Even detailed evaluation of hepatobiliary or pancreatic cancer requires about 10 minutes. IOUS-guided procedures are usually much faster and safer than “blind” procedures without IOUS.
IOUS is highly accurate compared to preoperative imaging studies or even to surgical exploration of deeper lesions or structures. Hepatic, endocrine, and other small tumors are often more accurately detected with IOUS. Vascular tumor invasion (e.g., portal vein invasion by pancreatic cancer) and the size of the lymph nodes are more precisely assessed. IOUS provides multi-planar images from multiple angles in real time, thus offering more imaging views and three-dimensional information than operative radiography. The unique capability of IOUS in guiding various procedures cannot
be substituted by intraoperative radiography. The appropriate use of IOUS can have a marked impact on intraoperative management, including improved decision making, reduction in surgical tissue dissection, operating time and complications, and development of new procedures.
be substituted by intraoperative radiography. The appropriate use of IOUS can have a marked impact on intraoperative management, including improved decision making, reduction in surgical tissue dissection, operating time and complications, and development of new procedures.
IOUS has limitations or disadvantages. Limitations in imaging capabilities relate to detectability of small lesions and delineation of ductal or tubular structures. IOUS displays smaller fields of view than operative radiography. Diagnosis and localization of fistulas is limited. Special transducers that are small and easily maneuverable in the operative field are required for IOUS scanning. Currently, each high-frequency IOUS probe costs in the range of $6,000 to $10,000. One disadvantage of IOUS is operator dependency. There is a learning curve for mastering IOUS; this will be solved by surgeon’s recognition of the benefits of IOUS, which should provide sufficient motivation to acquire the needed skills. We believe that these limitations or disadvantages are outweighed by the multiple advantages of IOUS.
Advantages and Limitations of Laparoscopic Ultrasound
The main advantage of LUS during laparoscopic exploration is the capability of compensating for the disadvantage of laparoscopy per se, particularly the loss of tactile sensation and the inability to examine deeply located structures or lesions. Overall, advantages of LUS over preoperative imaging studies and operative radiography are basically the same as those of IOUS. Likewise, limitations or disadvantages are similar.
Because of the same high-frequency instrument, the resolutions of LUS should be the same as that of IOUS. However, LUS scanning techniques are more difficult than IOUS scanning performed during laparotomy because of limited access to organs by LUS. For example, the complete LUS screening of the entire liver is not as easy as IOUS screening. Therefore, information and diagnostic accuracy provided by LUS should be close to, but may not be as accurate as those provided by open IOUS. The time needed to scan the same organ or lesions is longer with LUS. The learning curve for LUS is also longer. LUS probes are more costly and availability is still limited. LUS equipment needs some refinements, particularly in the development of an appropriate needle-guidance system, which currently is not widely available.
There are no confirmed biological effects on patients or examiners caused by present ultrasound equipment, including that used for IOUS and LUS. Although potential complications include organ injuries due to probe manipulation and contamination due to disruption of a sterile cover, such complications are very rare during diagnostic IOUS or LUS.
Instrumentation
The equipment of choice for IOUS and LUS is a so-called small parts scanner, which is a high-frequency high-resolution real-time ultrasound system, using transducers with frequencies of 5 to 10 MHz. While ultrasound at higher frequency does not penetrate deeply, it provides greater resolution of images; this is an ideal trade-off for IOUS/LUS because penetrating the body wall is not an issue. With a 7.5 MHz transducer, the sound penetration depth is about 8 to 10 cm, and small lesions such as 1-mm calculi and 3- to 5-mm tumors can be delineated. To meet special requirements of IOUS and LUS applications, several manufacturers have developed high-frequency probes specifically designed for use within the operative field.
Two basic shapes of IOUS probes are flat and cylindrical. Flat probes consist of linear-array or curvilinear-array (convex) transducers, and are usually configured into “T” or “I” shape (Fig. 1). T-shaped probes have either side- or end (front)- viewing. Cylindrical probes consist of curvilinear-array (convex) or phased-array transducers. They are pencil-like, often with flattened sides to achieve greater slimness. Flat probes are suitable for examination of relatively large, flat organs such as the liver, pancreas, and kidney. Flat side-viewing probes are particularly important for the scanning of the liver because the probe must be placed between the anterior surface of the liver and the diaphragm. On the other hand, cylindrical probes are useful for examination of small target organs such as the extrahepatic biliary duct, especially when areas of interest are situated deeply in the operative field where manipulation of the probe is limited. It is ideal to have both types of probes; however, a side-viewing flat T-shaped probe can be used for examination of abdominal organs in almost all abdominal operations.
LUS probes consist of transducers mounted on or near the tip of a slender shaft (Fig. 1). The shaft is usually 10 mm in diameter and 50 to 70 cm in length. Several types of LUS probes are presently available, providing linear-array, convex, or sector transducers. A probe can be either side viewing (scanning plane at the right angle with the probe shaft) or front viewing (scanning plane parallel to the shaft). A side-viewing linear-array or convex probe can be used for LUS scanning of almost all organs of the abdomen, and is essential for scanning of the liver. A front-viewing probe is suitable for scanning of the extrahepatic bile duct. A rigid-shaft probe and a flexible-tip probe are available. A flexible tip is mobile in two or four directions (bi- or quad-directional). A flexible tip facilitates scanning of areas that are difficult to delineate with a rigid probe (e.g., behind the dome of the liver), and may reduce the number of trocar insertion sites. LUS-guided needle placement may be more difficult with a side-viewing probe; this can be done much easier with a rigid front-viewing probe.
Scanning Techniques
Preparations and Timing of Scanning
In the operating room, usually, the person performing the scan should be in the surgeon’s position. The monitor should be placed to permit easy viewing of the screen and the operative field with minimal change-of-gaze movement. Probe sterility for intraoperative use is achieved by cold gas sterilization or use of a sterile cover. A newer sterilization method such as a low-temperature chemical sterilization, which can sterilize instruments in 30 to 60 minutes is available, and some probes are amenable to this method.
IOUS or LUS can be performed at any time and repeatable during the operation. Early in the course of operation, IOUS/LUS is used to obtain new information, which may help early operative decision making and determine the approach or the type of procedure to be performed. For example, immediately after laparotomy or laparoscopy for abdominal malignancy, IOUS/LUS can be conducted to evaluate the liver and lymph nodes as well as the primary lesion. IOUS/LUS can be used during surgical procedure for guidance. After finishing a procedure but prior to closure, completion IOUS/LUS examination can be performed to look for problems that still may be corrected. It is therefore, recommended to keep the IOUS/LUS equipment available and the probe sterile until closure so that IOUS/LUS scanning can be repeated whenever indicated.
Probe Placement
Ultrasound scanning is a two-step process: probe placement and probe movement. Various techniques can be performed to
facilitate IOUS/LUS scanning and to obtain the best imaging information. Two basic transducer placement techniques of IOUS/LUS are contact scanning and probe-standoff scanning. In contact scanning the probe is placed in direct contact with the tissue or organ, whereas in probe-standoff scanning the probe is positioned 1 to 2 cm away from the surface of structure (Fig. 2).
facilitate IOUS/LUS scanning and to obtain the best imaging information. Two basic transducer placement techniques of IOUS/LUS are contact scanning and probe-standoff scanning. In contact scanning the probe is placed in direct contact with the tissue or organ, whereas in probe-standoff scanning the probe is positioned 1 to 2 cm away from the surface of structure (Fig. 2).
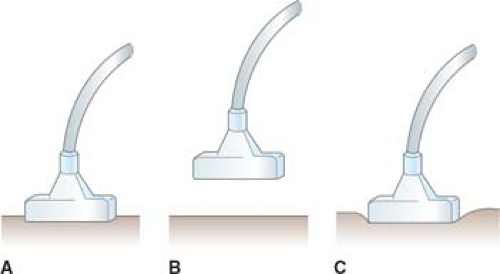 Fig. 2. Intraoperative ultrasound probe placement, including contact scanning (A), probe-standoff scanning (B), and compression scanning (C). |
The size of the target organ and the distance from the probe to the
area of interest in the organ determines which scanning technique should be used. Usually, contact scanning is used for examination of the interior of the liver, kidney, or pancreas (Fig. 3). However, when the surface or the superficial area of the organs needs to be examined, probe-standoff scanning should be employed. For examination of relatively small superficial structures such as the extrahepatic bile ducts, probe-standoff technique is particularly important (Fig. 4). In probe-standoff scanning, acoustic coupling is obtained between the tissue and the probe by filling the operative field with saline until the transducer surface of the probe is immersed beneath the solution. The probe-standoff with saline immersion technique is a unique IOUS/LUS method that is not commonly used in percutaneous ultrasound. This permits placement of the target at the appropriate focal distance and also prevents inadvertent compression of structures by the probe. One additional useful technique is compression scanning, in which the tissue is compressed intentionally by the probe (Fig. 2). This technique helps to eliminate air between the transducer and the tissue. Also, air in the gastrointestinal tract lumen that overlies a region of interest can be displaced by compression. This is especially effective when air in the duodenum obscures the distal common bile duct. Compression is also used to distinguish arteries from veins, which are more easily compressed.
area of interest in the organ determines which scanning technique should be used. Usually, contact scanning is used for examination of the interior of the liver, kidney, or pancreas (Fig. 3). However, when the surface or the superficial area of the organs needs to be examined, probe-standoff scanning should be employed. For examination of relatively small superficial structures such as the extrahepatic bile ducts, probe-standoff technique is particularly important (Fig. 4). In probe-standoff scanning, acoustic coupling is obtained between the tissue and the probe by filling the operative field with saline until the transducer surface of the probe is immersed beneath the solution. The probe-standoff with saline immersion technique is a unique IOUS/LUS method that is not commonly used in percutaneous ultrasound. This permits placement of the target at the appropriate focal distance and also prevents inadvertent compression of structures by the probe. One additional useful technique is compression scanning, in which the tissue is compressed intentionally by the probe (Fig. 2). This technique helps to eliminate air between the transducer and the tissue. Also, air in the gastrointestinal tract lumen that overlies a region of interest can be displaced by compression. This is especially effective when air in the duodenum obscures the distal common bile duct. Compression is also used to distinguish arteries from veins, which are more easily compressed.
Probe Movement (Scanning Maneuvers)
The second step in ultrasound scanning is probe movement. A target or suspected lesion should be scanned from various positions and directions, and an organ should be scanned in a systematic fashion for a thorough IOUS/LUS examination. Although the scanning method varies depending on the organ examined, it is usually important to obtain longitudinal, transverse, and at times, oblique views of a lesion or organ (Fig. 5). A longitudinal view is the ultrasound image parallel to the long axis of the structure or organ being examined, whereas a transverse view is the image at the right angle to the long axis. Oblique views are intermediate in position between longitudinal and transverse views.
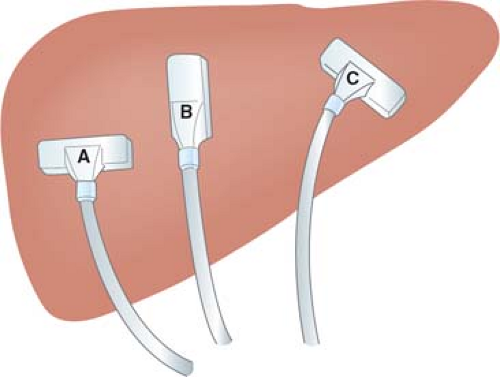 Fig. 5. Transverse (A), longitudinal (B), and oblique (C) scanning of the liver. One probe can be used to perform scanning of these planes by “rotating” maneuver. |
Scanning maneuvers are the ways by which the probe is manipulated during its movement. There are three basic scanning maneuvers of the probe: sliding, rotating, and angulating (rocking or tilting). In “sliding,” the probe slides across the surface of a tissue or organ while the probe-to-surface geometry is maintained. In “rotating,” the probe is turned along the direction of the sound beam, either
clockwise or anticlockwise. With this technique, lesions or structures are delineated continually from longitudinal to oblique to transverse views or vice versa, and thus three-dimensional (3D) information of lesions can be acquired. In “angulating,” the probe’s head (transducer surface side) remains relatively stationary while the shaft of the probe is moved or swung to different angles (Fig. 6). This technique is especially useful in a deep and restricted operative field where “sliding” of the probe is limited or impossible. These basic maneuvers can be combined simultaneously, and in reality, combination maneuvers are commonly performed during IOUS.
clockwise or anticlockwise. With this technique, lesions or structures are delineated continually from longitudinal to oblique to transverse views or vice versa, and thus three-dimensional (3D) information of lesions can be acquired. In “angulating,” the probe’s head (transducer surface side) remains relatively stationary while the shaft of the probe is moved or swung to different angles (Fig. 6). This technique is especially useful in a deep and restricted operative field where “sliding” of the probe is limited or impossible. These basic maneuvers can be combined simultaneously, and in reality, combination maneuvers are commonly performed during IOUS.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree