Tumors of the Urinary Tract in Urine and Brushings*
TUMORS OF THE UROTHELIUM (TRANSITIONAL EPITHELIUM) OF THE BLADDER
Epidemiology
In the United States, tumors of the bladder are the fourth leading type of cancer in men but are less common in women (Messing and Catalona, 1998). During the second half of the 20th century, a statistically significant increase in the rate of urothelial tumors, mainly tumors of the urinary bladder, has been observed in most industrialized countries
(Cole et al, 1971, 1972; Wynder et al, 1977; Silverman et al, 1992). For the year 2001, the American Cancer Society projected more than 54,000 new cases and 12,400 deaths from tumors of the bladder (Greenlee et al, 2001).
(Cole et al, 1971, 1972; Wynder et al, 1977; Silverman et al, 1992). For the year 2001, the American Cancer Society projected more than 54,000 new cases and 12,400 deaths from tumors of the bladder (Greenlee et al, 2001).
The impact of environmental factors on the genesis of tumors of the bladder has been known since the publications by the German surgeon Rehn (1895 and 1896), who observed that workers in factories producing aniline dyes were at a high risk for this disease. It was subsequently shown that the carcinogenic compounds to which these workers were exposed were aromatic amines, such as 2-naphthylamine, para-aminodiphenyl (xenylamine), and 4-4′-diaminobiphenyl (benzidine) (Bonser et al, 1952; Boyland et al, 1954). Another compound known as MBOCA [4,4′ methylenobis (2-chloroaniline)], an analogue of benzidine, has been shown to induce low-grade papillary tumors in the bladder (Ward et al, 1988). The drug chlornaphazine, related to the aromatic compounds, was shown to be carcinogenic for the bladder (Videbaek, 1964; Laursen, 1970). The effects of the alkylating agent cyclophosphamide as a carcinogen in the lower urinary tract were extensively discussed in Chapter 22. Women working in factories producing phenacetin, a common analgesic, and heavy users of the drug are also at increased risk for urothelial tumors that may involve the bladder but also the ureters and the renal pelves (Johansson et al, 1974; Mihatsch, 1979; Lomax-Smith, 1980; Piper et al, 1985). There also is evidence that workers in rubber and cable, leather, and shoe repair industries are at a high risk for bladder cancer, although the specific carcinogenic substances have not been clearly identified. Nortier et al (2000) reported that the use of a Chinese herb (aristolochia fangchi) may also be a risk for bladder tumors. Along similar lines, bladder tumors in cattle have been linked with consumption of another plant, bracken fern (pteris aquilina) (Pamukcu et al, 1964; Hirono et al, 1972). Experimental data suggested that bladder tumors in cattle fed bracken fern may also be associated with bovine papillomavirus type 2 (Campo et al, 1992). A high level of inorganic arsenic in drinking water is another cause of bladder cancer (Cohen et al, 2000; Steinmaus, et al, 2000). Bladder cancer is by far the most common tumor in the population at risk, although organs such as the lungs may also be affected. Bladder tumors observed in Taiwan in areas of high arsenic concentration, are commonly associated with arteriosclerotic changes in lower extremities known as the “black foot” disease (Chiang et al, 1993; Chiou et al, 1995, 2001). The association has also been observed in Chile (Smith et al, 1998) and Argentina (Hopenhayn et al, 1996). The mechanisms of arsenic carcinogenicity are unknown (Simeonova and Luster, 2000).
Besides the environmental factors, there are other risk factors for tumors of the bladder. For example, paraplegic and quadriplegic patients are at risk, presumably because of inadequate voiding, and therefore exposure of the bladder to small doses of unknown carcinogenic agents contained in the urine (Kaufman et al, 1977; Bejany et al, 1987; Bickel et al, 1991). Similar mechanisms may be responsible for bladder tumors in otherwise normal men with low intake of fluids (Michaud et al, 1999) and enlargement of the prostate.
Work from this laboratory has shown that prostatic enlargement, whether caused by hyperplasia or carcinoma, is another risk factor for cancer of the bladder. Between January 1974 and August 1977, we observed 19 patients, seen primarily because of prostatic enlargement, whose urinary sediment disclosed an occult urothelial carcinoma, subsequently confirmed by biopsies of bladder. Further review of the files at Montefiore Medical Center, compiled by Dr. Allayne Kahan, disclosed 13 patients with coexisting carcinomas of the prostate and of the bladder and an additional 28 patients with benign prostatic hypertrophy and bladder cancer (unpublished data). Barlebo and Sørensen (1972) observed 2 patients with carcinoma in situ of the bladder, initially seen because of prostatic hypertrophy. A further association of bladder cancer with prostatic disease was reported by Mahadevia et al (1986), also from this laboratory. Mapping of 20 cystoprostatectomy specimens removed because of invasive high-grade bladder cancers or carcinoma in situ, or both, disclosed that occult carcinoma of the prostate was present in 14 of the 20 patients, but only one of these lesions was suspected before cystectomy. These observations strongly suggest that in all patients with prostatic enlargement, whether benign or malignant, bladder cancer should be ruled out. In fact, Nickel et al (2002) reported that three urothelial carcinomas in situ were observed among 150 patients with chronic prostatitis evaluated by urine cytology. Conversely, male patients with known tumors of the bladder should be investigated for coexisting prostatitic carcinoma. The urologists are generally unaware of this association.
Tumors of the bladder are observed with high frequency in some geographic areas. In the United States, these tumors are often observed in the state of New Jersey and in New Orleans, presumably because of a high level of exposure to industrial waste. In Egypt and many other African countries, an infection with the parasite Schistosoma haematobium (Bilharzia) is an important cause of bladder cancer, as discussed in Chapter 22 and in this chapter. Still, many patients with bladder tumors have no known risk factors. It is speculated that industrial pollution, cigarette smoking, or a combination of these and other yet unknown factors contribute to cancers of the lower urinary tract.
Terminology
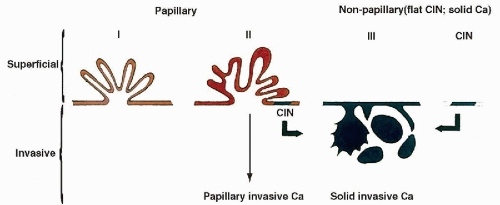
The unique features of the epithelium lining the lower urinary tract were discussed at length in Chapter 22 and need not be repeated here. Many of these features, such as the presence of the asymmetric unit membrane and umbrella cells, are observed in tumors derived from this epithelium. Further, the presence of uroplakins, proteins uniquely characterizing this epithelium (summaries in Wu et al, 1994 and Sun et al, 1999), have been shown to be an important diagnostic and experimental tool, as discussed elsewhere in this chapter. For all these reasons, the term urothelial tumors or carcinomas has been used in the previous additions of this book and in other writing, replacing the old term transitional cell tumors or
carcinomas (Koss, 1974, 1985, 1995). The term urothelial tumors has now been accepted by consensus of urologic pathologists (Epstein et al, 1998).
carcinomas (Koss, 1974, 1985, 1995). The term urothelial tumors has now been accepted by consensus of urologic pathologists (Epstein et al, 1998).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree