Tumor Metastases in Lymph Nodes
Partmark Metastatic Tumors in Lymph Nodes
The involvement of lymph nodes by metastatic tumors signifies the start of a new phase in the progreWss of a cancer. It indicates that through a succession of molecular changes, the cancer cells have acquired phenotypes that enable them to invade, colonize, and disseminate.
Establishing the presence of metastatic tumor in lymph nodes is essential for the management and prognosis of cancer. In human solid cancer, lymph node status is the most important indicator of clinical outcome (1). The vast majority of lymph node biopsies, particularly those on frozen sections, are performed to confirm or exclude tumor metastases. The anatomic location and the number of lymph nodes involved are also important indicators of the process, and new methods, such as sentinel lymph node biopsies, have been devised to answer questions of staging. Not infrequently, a lymph node metastasis is discovered before an occult primary tumor is detected. In such cases, to identify the unknown primary tumor, extensive studies of the lymph node metastases, including immunohistochemistry and electron microscopy in addition to detailed histopathology, are often necessary.
In the following 13 chapters, the diagnostic problems relating to the most co mmon tumor metastases of lymph nodes are examined.
Pathobiology Of Tumor Metastasis
The major cause of the morbidity and mortality associated with tumors is metastasis. Although substantial progress has been achieved in the early diagnosis and treatment of malignant tumors, mechanisms of dissemination remain largely unknown. The elucidation of such mechanisms is essential to prevent metastases and cure cancer. Tumor metastasis is a complex phenomenon based on a cascade of interdependent events: detachment of cells from the primary tumor, advancement through the tumor matrix, penetration through the basement membranes of lymphatics and blood vessels, circulation through the vascular flow, arrival in remote organs, and formation of independent colonies with their own growth factors and vascular supply (2). Some believe that primary tumors are largely heterogeneous and so may include cells endowed with a capacity for growth autonomy and metastasis (3). Others think that the potential for metastasis arises through mutations, with new variants generated and selected during tumor development (4).
The fact that not all malignant tumors are metastatic suggests that the metastatic phenotype is different from and independent of the tumorigenic phenotype (5,6). The multiple steps in the metastatic cascade of events are controlled at the genetic level through activation or deactivation of multiple genes. As counterparts of the genes promoting the formation of metastatic phenotypes, metastatic suppressor genes degrade the gene products that favor tumor cell dissemination (7). Because malignant cells are inherently unstablex, their genomes are subject to constant variation, resulting in what is termed phenotypic drift (8). During cancer development accumulation of genetic changes modulates the sensitivity of neoplastic cells to invasion stimulators and invasion inhibitors in a newly acquired invasive phenotype (9,10,11). Various genes undergo irreversible structural changes (mutations), such as deletions, amplifications, and translocations, which yield altered gene products (8). Among them are adhesion molecules such as E-cadherins, the downregulation of which decreases tumor cell cohesiveness; integrins, which determine the affinity of tumor cells for basement membranes; and proteolytic enzymes, such as type IV collagenase, which are essential for tumor cell invasiveness (7,9,10). A particular adhesion molecule, CD44, and its variants, which are normally expressed on T lymphocytes, has also been detected on some tumor cells, which suggests a possible role of T lymphocytes in tumor implantation in lymph nodes (11). Thus, tumor cells progressively acquire the characteristics necessary for invasion and metastasis that characterize the metastatic phenotype.
Tumor cells and tumor-infiltrating lymphocytes (TIL), both in the primary tumor and in its metastases are engaged in reciprocal death-inducing activities. The interactions of tumor cells and TIL may result in the apoptosis of either cells. One system involved in the process of apoptosis is that of FAS, a member of the tumor necrosis family and its ligand FAS-L. We demonstrated the coexpression of FAS and FAS-L by both breast carcinoma cells and their TIL (12). Subsequently we recorded and compared their expression on the primary breast tumors and their lymph node metastases and noted that in the former, FAS was stronger than FAS-L while the reversal, FAS-L stronger than FAS, was observed in the metastases. These results are consistent with the interpretation that the upregulated expression of FAS-L in the metastatic tumor cells is related to their ability to defend against anti-tumor immune attack by TIL in the lymph nodes. It is understood that although essential for apoptosis, the FAS/FAS-L system is only a contributing factor in the complex process of tumor invasion and anti-tumor defense (12). Tumor cells progressively acquire the characteristics necessary for invasion and metastasis, which characterize the metastatic phenotype. Consequently, the success or failure of a tumor to invade and metastasize depends on the interaction of gene products released by stimulatory and inhibitory metastasis genes (6).
Tumor Metastasis In Lymph Nodes
Lymph nodes play an essential role in the control of tumor progression. In response to the antigenicity of tumor cells, regional lymph nodes may initiate and develop complex immune reactions. At the same time, they may entrap circulating tumor cells that have originated in their tributary territories. acting as efficient barriers, the lymph nodes may be able to destroy invading tumor cells completely, or at least stop their dissemination temporarily. Lymph node metastasis, in contrast to the vascular spread of tumors, presents an opportunity, even if temporary, for surgical intervention. In addition, because of their accessibility, Lymph nodes with metastatic tumor present the best opportunity for primary tumor diagnosis through biopsy and histologic evaluation.
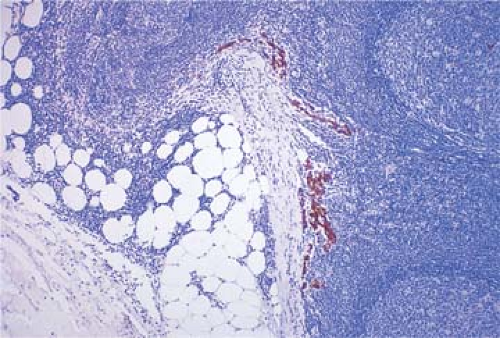
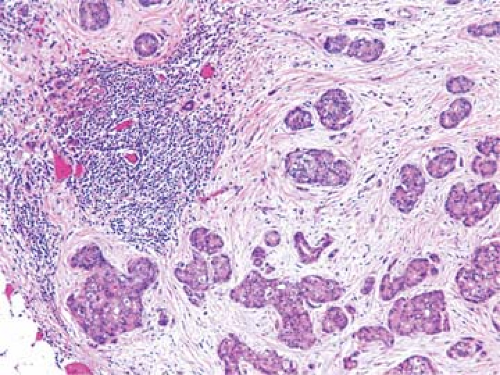
Metastatic tdumor cells first appear in the marginal sinus (Fig. 86.1), from which they penetrate the medullary sinuses, medulla, and cortex; the eventual result is total parenchymal replacement. Even before metastatic tumor cells are present in the lymph node, reactive changes take place which reorganize favorably the microenvironment (13,14) (see Chapter 44). Newly discovered vascular endothelial factors (VEGF-C and VEGF-D promote tumor-associated lymphangiogenesis which increase the proliferation of endothelial cells in high endothelial venuels resulting in blood- and lymph-enriched lymph nodes (13,14). Within the lymph nodes, the tumor cells may induce various tissue reactions, such as reactive follicular hyperplasia (Fig. 86.2), sinus histiocytosis, angiogenesis (Fig. 86.3), and foreign-body granuloma formation Fig. 86.4). Some metastatic tumors elicit intense desmoplastic reactions, which contribute to the partial or total replacement of lymphoid tissues (Fig. 86.5). Finally, radiotherapy or chemotherapy may wipe out lymphoid tissue with the tumor and create an unusual histologic appearance of the lymph nodes affected (Fig. 86.6). Prior involvement of a lymph node by leukemia or lymphoma
does not preclude the subsequent growth of a metastatic tumor (Fig. 86.7).
does not preclude the subsequent growth of a metastatic tumor (Fig. 86.7).
 Figure 86.1. Metastatic poorly differentiated mammary ductal carcinoma progressing from marginal Dsinus into cortical sinus of axillary lymph node. HPS stain. |
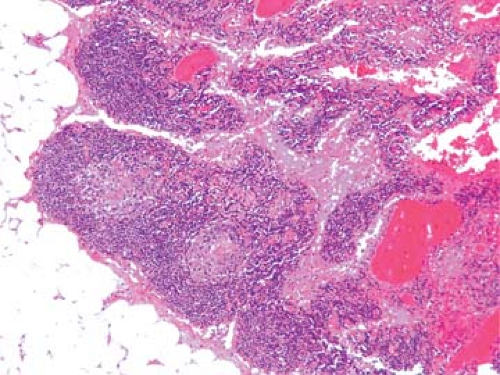 Figure 86.3. Axillary lymph node reactive to mammary carcinoma with follicular hyperplasia, markedly distended sinuses and angiogenesis. HPS stain. |
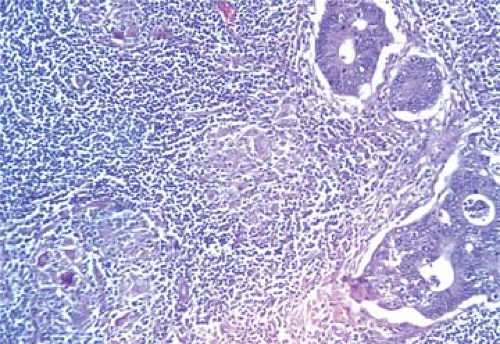 Figure 86.4. Lymph node metastasis of adenocarcinoma accompanied by foreign body giant cell granulomas. HPS stain. |
Staging And Prognosis Of Tumors
Cancer management and prognosis depend to a great extent on the presence and degree of tumor metastasis. These are evaluated by staging tumors according to the internationally accepted tumor-node-metastasis (TNM) system. Of all the various criteria used as prognostic factors, the most powerful remains the description of anatomic spread according to the TNM formula (15,16). This classification by stage is as follows: stage 0, preinvasive neoplasia; stage I, tumor confined to the organ of origin; stage II, direct tumor spread outside the organ of origin; stage III, metastasis to regional lymph nodes; and stage IV, metastasis to distant sites. Each successive stage in the TNM system indicates a significant decrement in the prognosis. The diagnosis of lymph node tumor metastasis is therefore essentially important for cancer therapy. This consists not only of establishing the presence of lymph node metastasis but
also of evaluating the site of the primary tumor and its degree of histologic differentiation and determining the tumor cell phenotype and prognostic indicators of tumor cell behavior.
also of evaluating the site of the primary tumor and its degree of histologic differentiation and determining the tumor cell phenotype and prognostic indicators of tumor cell behavior.
Topography Of Involved Lymph Nodes
The anatomic location of a lymph node involved with metastatic tumor provides an important indication of the site of the primary tumor (Table 86.1).
Cervical lymph nodes, particularly high jugular and posterior cervical nodes, drain the head and neck and may harbor metastatic carcinomas originating in the nasopharynx (see Chapter 89), tonsillar fossa, tongue, floor of the mouth, thyroid (see Chapter 90), extrinsic larynx, facial skin, and scalp.
Scalene lymph nodes representing the lower, deep jugular chain are commonly the site of metastases from intrathoracic carcinomas, particularly in the lungs. The left scalene lymph nodes also drain metastases of intraabdominal tumors.
TABLE 86.1 MOST COMMON PRIMARY ORIGINS OF REGIONAL LYMPH NODE METASTASES | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
 Figure 86.8. Metastatic gastric adenocarcinoma in supraclavicular lymph node (Virchow node). HPS stain. |
The supraclavicular lymph nodes are most often the site of metastases of abdominal cancers. The left supraclavicular lymph node, frequently invaded by gastric carcinoma, is classically known as the Virchow node (Fig. 86.8).
 Figure 86.7. Metastatic breast carcinoma in lymph node with small lymphocytic lymphoma/chronic lymphocytic leukemia. HPS stain. |
In women, involvement of the axillary lymph nodes with metastatic carcinoma generally indicates the presence of ipsilateral breast cancer and its assessment is essential in the staging of the tumor. The sentinel lymph node biopsy has become the standard procedure for the evaluation of lymph node metastasis (see Chapter 88). Melanomas and squamous cell carcinomas of the upper extremities and trunk are the next most frequent primary tumors to invade the axillary nodes (see Chapter 87).
Inguinal lymph node metastases in 2,232 patients were reviewed by Zaren and Copeland (17), and the locations of the primary tumors, determined in 99% of the cases, were in descending order of frequency as follows: skin of the lower extremities, cervix, vulva, skin of the trunk, rectum and anus, ovary, and penis.
Pelvic lymph nodes may be the site of metastases from the prostate and testes, the female genital tract, and the lower extremities.
The topographic correlations mentioned here are helpful clues to the most common sites of origin of metastases in various groups of regional lymph nodes. Notwithstanding the anatomic connections between the location of neoplasms and their draining lymph nodes, metastatic tumors can occasionally be found in unexpected and remote areas. Because of blockage
of lymphatics by tumor cells, with subsequent retrograde flow and circulation shunts, paradoxical metastases, contralateral metastases, and metastases of deep-seated tumors in peripheral lymph nodes may be encountered. Not unlike hematogenous metastases, the involvement of lymph nodes by secondary tumors can vary greatly. Therefore, although the most common sites of origin for each regional group of lymph nodes should be considered first, all other possible locations of primary tumors must be systematically reviewed in every case.
of lymphatics by tumor cells, with subsequent retrograde flow and circulation shunts, paradoxical metastases, contralateral metastases, and metastases of deep-seated tumors in peripheral lymph nodes may be encountered. Not unlike hematogenous metastases, the involvement of lymph nodes by secondary tumors can vary greatly. Therefore, although the most common sites of origin for each regional group of lymph nodes should be considered first, all other possible locations of primary tumors must be systematically reviewed in every case.
Lymph Node Metastases Of Occult Primary Tumors
In general, a statistical relationship exists between tumor size and the incidence of metastases. Thus, it has been clearly established that in carcinoma of both breast and colon, the probability of finding metastases in regional lymph nodes increases in proportion to tumor volume (18). However, important exceptions to these correlations include large tumors that do not metastasize and very small tumors that disseminate widely. To a large extent, the capacity for metastasis represents an intrinsic quality of tumor cells that in most cases correlates inversely with the degree of cellular differentiation. During tumor progression, the capacity for metastatic dissemination of tumor cells may change and, within each tumor, because of genomic instability and frequent mutations, new populations of tumor cells with increased metastatic potential may gradually emerge (19). Sometimes, lymph node metastases may occur without a detectable primary tumor as the result of enhancement of the metastatic potential of tumor cells and regression of the primary neoplasm, an event known to happen occasionally, particularly with melanoma (20,21) and seminoma (22,23) (see Chapters 87 and 92).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree