Cytopathology
Role of Touch Imprints/Smears in Lymph Node Diagnosis
The histologic examination of lymph node biopsy specimens allows for the assessment of both architecture and cytology. Assessment of architecture is obviously important but, in the evaluation of lymphomas, cytologic assessment is equally or even more important. The assessment of lymphoma cell cytology, as assessed by the study of well-fixed, well-prepared thin histologic sections at high-power magnification, played an essential role in understanding lymphocyte biology and in the establishment of earlier lymphoma classification systems (e.g., Lukes-Collins, Kiel).
Another extremely helpful approach to cytologic examination is the preparation and examination of touch imprints and smears as a part of the assessment of lymph node or tissue biopsy specimens involved by lymphomas (1). These are easily prepared from unfixed lymph nodes received in the laboratory for frozen section or as soon as possible after excision, and should be performed routinely. Diagnostic imprints and smears in some cases can preclude the need for frozen section, thereby saving tissue for routine diagnosis, ancillary studies, or tissue banking. Imprints and smears can be quickly fixed and stained with Papanicolaou and hematoxylin-eosin stains or they can be air-dried and stained with Romanowsky-based stains (Diff-Quick or Wright-Giemsa stains). Ideally, multiple, differently stained slides should be examined. For hematopathologists more comfortable with tissue biopsy specimen analysis, the cytologic features of fixed slides are similar to those seen in tissue sections. For hematopathologists more comfortable with peripheral blood and bone marrow specimens, the cytologic features of air-dried slides are similar to blood and bone marrow smears. Romanowsky-based stained slides are advantageous because they stain cellular granule content, and are particularly helpful in the recognition of basophils and mast cells because they highlight their purple granules. Unstained air-dried imprints and smears also should be saved for cytochemical and immunocytochemical staining, or molecular studies, if needed. Cytochemical stains can be very helpful in the assessment of dysplasia in specimens derived from patients with myelodysplastic or myeloproliferative disorders. Cytochemistry is also helpful in the typing of myeloid sarcoma (e.g., granulocytic versus monocytic). Immunohistochemistry is helpful in establishing cell lineage, essential for the classification of non-Hodgkin lymphomas.
It must be remembered that cytologic diagnostic criteria are different from those applied to tissue sections, and that the study of lymph node imprints must be performed routinely to gain experience and achieve proficiency. Examination of touch imprints and smears is also very helpful training that can be applied to the examination of fine needle aspiration specimens.
Touch Imprints
Technique
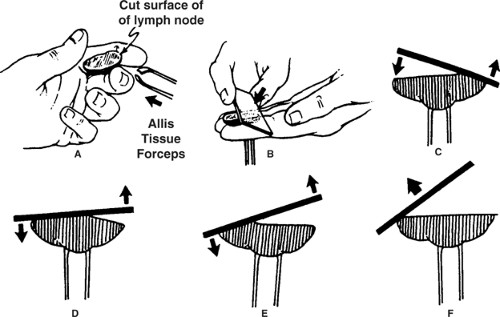
Well-prepared touch imprints of lymph nodes (or other biopsy specimens) should display a large, cellular monolayer that yields both architectural and cytologic information (1). One method to achieve this goal is to lightly press a fresh slice of lymph node against a clean, uncoated glass slide. Alternatively, smoothly roll a glass slide, at constant speed and without pulling at the end, over a cut, firmly held lymph node (2) (Fig. 3.1). Sideways shearing disrupts fragile cells, exploding the lymphocytes into a streak of elongated bare nuclei (“blue spaghetti”). Before contacting the slide, carefully blot or wipe excess blood or serum from the cut tissue. Make several touch imprints using different pressures to find an optimal preparation, especially for lymph nodes with sticky, watery, or excessively dry surfaces (2). Touch imprints to be stained with Papanicolaou or hematoxylin-eosin stains must be fixed immediately, to avoid air-drying artifact. Touch imprints to be stained with Wright-Giemsa or Diff-Quik must be completely dried before staining, to avoid staining artifact related to residual moisture.
Diagnostic Value
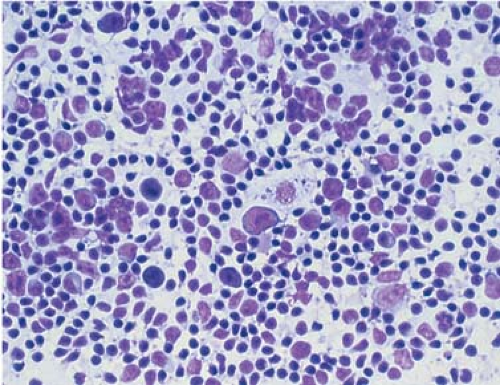
Lymph node touch imprints are suitable for immunohistochemical, morphometric, and DNA ploidy analysis (3,4,5,6). However, touch imprints (in comparison with cytocentrifuge smears prepared from cell suspensions) introduce selective bias by underrepresenting large lymphoid cells, revealing fewer nuclear irregularities, and poorly preserving cytoplasmic borders (6). Nevertheless, because of their ease of preparation and reasonably good morphologic preservation, touch imprints are valuable for rapid intraoperative diagnosis (7). In a study from Nigeria, pathologists without quick access to histologic slides evaluated lymph nodes by touch imprint; they could accurately classify Burkitt lymphoma, Hodgkin lymphoma (HL), and metastatic carcinoma (sensitivity, 85% to 100%), but not other types of non-Hodgkin lymphoma, chronic lymphadenitis, or tuberculosis (sensitivity, 31% to 50%) (8). In addition to allowing one to evaluate the cytologic features of the cells, some architectural information also can be derived. For example, in nodular processes such as reactive follicular hyperplasia and follicular lymphoma, the touch imprint can highlight aggregates of cells corresponding to the follicles (Fig. 3.2).
Fine Needle Aspiration Biopsy
Indications
Fine needle aspiration biopsy (FNAB) of lymph nodes is an extremely useful procedure that can provide important
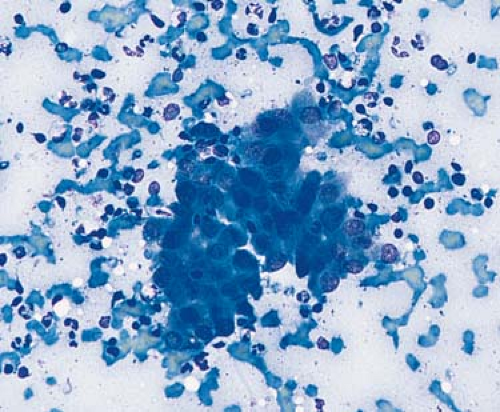
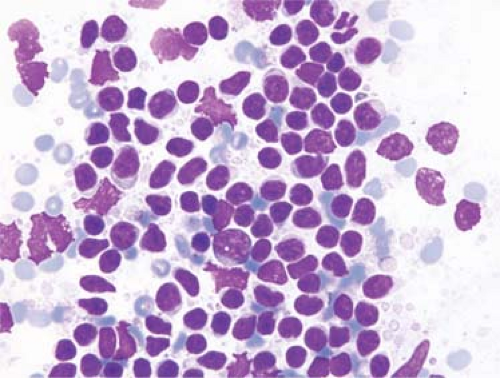
information that guides patient management while being minimally invasive. When is FNAB of lymph nodes indicated? As stated by Frable, “Unless a clinical problem is significant enough to warrant consideration of open surgical biopsy, aspiration biopsy should not be considered either” (9). In general, the diagnostic accuracy of FNAB is lower for lymphomas than for metastatic tumors (10,11). In many large series, FNAB of lymph nodes involved by metastatic carcinoma achieved a sensitivity of more than 90% (11,12). This high sensitivity is possible because the metastatic carcinoma cells are usually abundant, and their cytologic features are dissimilar to that of the cells of normal or hyperplastic lymph nodes (9,10,11) (Fig. 3.3). High sensitivity is also possible for many other types of metastatic neoplasms (e.g. sarcomas, melanoma) for similar reasons.
information that guides patient management while being minimally invasive. When is FNAB of lymph nodes indicated? As stated by Frable, “Unless a clinical problem is significant enough to warrant consideration of open surgical biopsy, aspiration biopsy should not be considered either” (9). In general, the diagnostic accuracy of FNAB is lower for lymphomas than for metastatic tumors (10,11). In many large series, FNAB of lymph nodes involved by metastatic carcinoma achieved a sensitivity of more than 90% (11,12). This high sensitivity is possible because the metastatic carcinoma cells are usually abundant, and their cytologic features are dissimilar to that of the cells of normal or hyperplastic lymph nodes (9,10,11) (Fig. 3.3). High sensitivity is also possible for many other types of metastatic neoplasms (e.g. sarcomas, melanoma) for similar reasons.
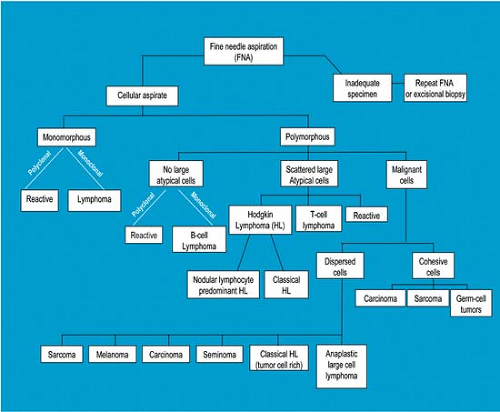
In the diagnosis of lymphomas, for many years FNAB has been accepted for the diagnosis of recurrent disease, staging, or obtaining additional material for immunophenotyping in patients with a diagnosis established by excisional biopsy and histologic examination. In recent years, FNAB of lymph nodes supplemented by ancillary studies has been increasingly accepted as an approach for primary diagnosis of reactive lymphoid lesions and lymphomas (Fig. 3.4) (13,14). This argument is made stronger for patients in whom the site of disease is not easily accessible, such as the retroperitoneum, because diagnostic FNAB results can preclude the need for excisional biopsy (15). In addition, in patients in whom the FNAB results are not diagnostic, excisional lymph node biopsy can then be
performed. In this scenario, there is little negative consequence to performing FNAB first, as fewer than 10% of previously aspirated lymph nodes show morphologic sequelae, such as a linear tract, hemorrhage, organization, and, rarely, myofibroblastic proliferation or infarction (16).
performed. In this scenario, there is little negative consequence to performing FNAB first, as fewer than 10% of previously aspirated lymph nodes show morphologic sequelae, such as a linear tract, hemorrhage, organization, and, rarely, myofibroblastic proliferation or infarction (16).
Morphologic Caveats
In this section, the discussion is focused on morphologic findings and caveats. It is recognized that immunophenotyping, discussed in the next section (and in Chapters 6 and 7), can resolve or reduce the number of morphologically problematic cases. In addition, the diagnosis of lymphoma is incomplete without an immunophenotypic assessment of cell lineage, as this is required in the current World Health Organization (WHO) lymphoma classification system and for assignment of appropriate therapy. Thus, if being used as an alternative to excisional biopsy and histologic examination, FNAB should never be performed without ancillary studies for immunophenotypic assessment, using either flow cytometry or immunocytochemistry. Molecular studies are also helpful in a subset of cases.
Reactive lymphoid lesions and hematologic neoplasms are distinguished from metastatic tumors because the cells are generally not cohesive. In general, lymphoid cells are also smaller than the cells of metastatic carcinomas and sarcomas. Cytoplasmic fragments (so-called lymphoglandular bodies and also known as Soderstrom bodies) occur more abundantly in smears of benign and malignant lymphoid disorders than in smears of nonlymphoid round-cell neoplasms (17). Cytoplasmic fragments are most numerous in B-cell lesions, but also occur in T-cell lesions and neoplasms of myeloid lineage (18).
Distinguishing between reactive lymphadenopathies and large-cell non-Hodgkin lymphoma is usually possible by morphologic assessment. In reactive lymphadenopathies, a mixture of cell types is present, including small and larger lymphocytes, tingible-body macrophages, granulocytes, and plasma cells in variable proportions (Fig. 3.5). In contrast, large-cell lymphomas are commonly composed of a relatively homogeneous population of cytologically atypical large cells that may have centroblastic (large noncleaved) (Fig. 3.6) or immunoblastic cytologic features (5,11,19). Mitotic figures and apoptotic cells are usually easily identified. Necrosis may be present. Similarly, high-grade non-Hodgkin lymphomas also can be distinguished morphologically from reactive lesions. In Burkitt lymphoma, the neoplastic cells are monotonous and intermediate in size (Fig. 3.7). In Wright-Giemsa–stained slides, the cells have many cytoplasmic vacuoles. Mitotic figures, apoptotic cells, and necrosis are common and, in some cases, most of the cells can be apoptotic (11,20). In cases of lymphoblastic lymphoma, the neoplastic cells are monotonous and small, approximately 1.5 times the size of reactive lymphocytes. Lymphoblasts have immature, blastic chromatin and either round or convoluted nuclear contours. Mitotic figures are usually numerous (21,22).
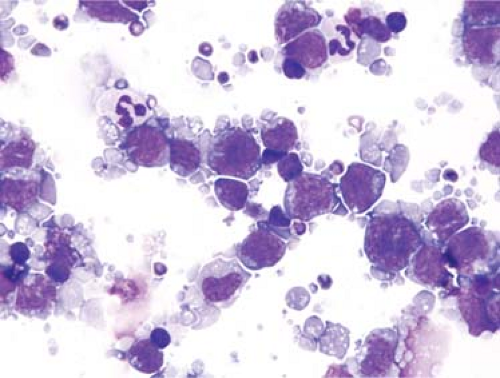 Figure 3.6. Fine needle aspiration biopsy specimen of lymph node involved by large B-cell lymphoma, shown to be of B-cell lineage by flow cytometry immunophenotyping. Wright-Giemsa stain. |
Distinguishing reactive lymph node lesions from peripheral T-cell lymphoma can be difficult in a subset of cases (11,23). In particular, types of peripheral T-cell lymphoma in which the neoplastic cells represent only a minority of cells in the specimen can be problematic. Angioimmunoblastic T-cell lymphoma is perhaps the best example. Immunophenotyping also may not be helpful in this differential diagnosis as clonal markers of T-cells (analogous to κ and λ in B-cells) do not exist, and the neoplastic cell population can be small and difficult to identify cytologically or gate by flow cytometry. Cases of peripheral T-cell lymphoma that are relatively monotonous and
composed of intermediately sized large cells can be recognized morphologically as lymphoma (23).
composed of intermediately sized large cells can be recognized morphologically as lymphoma (23).
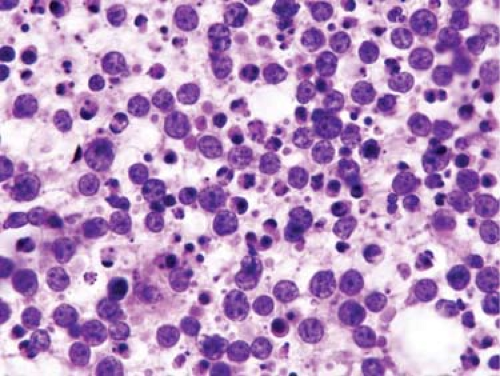 Figure 3.7. Touch imprint of lymph node with Burkitt lymphoma. Numerous apoptotic cells and necrotic debris are present. Hematoxylin-eosin stain. |
Distinguishing between reactive lymphadenopathies and low-grade B-cell lymphomas can be achieved morphologically in many cases, but classification is often is not possible by morphologic examination alone (24). In reactive lymphadenopathies, as stated earlier, a mixture of cell types exists. The lymphocytes have generally round nuclear contours. In contrast, low-grade B-cell lymphomas are composed of a relatively monotonous population of lymphoid cells (Fig. 3.8). In some types of low-grade B-cell lymphoma, the neoplastic cells have irregular nuclear contours (e.g., follicular lymphoma) or characteristic cell types (e.g., prolymphocytes and paraimmunoblasts in small lymphocytic lymphoma/chronic lymphocytic leukemia). However, it must be emphasized that some types of low-grade B-cell lymphoma are composed of a heterogeneous mixture of cells (e.g., marginal-zone B-cell lymphomas) and are more difficult to recognize (Fig. 3.9) (25,26). Immunophenotyping is essential for precise classification of low-grade B-cell lymphomas (10,24,26).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree