Tularemia is a zoonosis caused by Francisella tularensis. Humans of any age, sex, or race are universally susceptible to this systemic infection. Tularemia is primarily a disease of wild animals and persists in contaminated environments, ectoparasites, and animal carriers. Human infection is incidental and usually results from interaction with biting or blood-sucking insects, contact with wild or domestic animals, ingestion of contaminated water or food, or inhalation of infective aerosols. The illness is characterized by various clinical syndromes, the most common of which consists of an ulcerative lesion at the site of inoculation, with regional lymphadenopathy and lymphadenitis. Systemic manifestations, including pneumonia, typhoidal tularemia, meningitis, and fever without localizing findings, pose a greater diagnostic challenge.
ETIOLOGY AND EPIDEMIOLOGY
F. tularensis is a class A bioterrorism agent (Chap. 261e). With rare exceptions, tularemia is the only disease produced by F. tularensis—a small (0.2 μm by 0.2–0.7 μm), gram-negative, pleomorphic, nonmotile, non-spore-forming bacillus. Bipolar staining results in a coccoid appearance. The organism is a thinly encapsulated, nonpiliated strict aerobe that invades host cells. In nature, F. tularensis is a hardy organism that persists for weeks or months in mud, water, and decaying animal carcasses. Dozens of biting and blood-sucking insects, especially ticks and tabanid flies, serve as vectors. Ticks and wild rabbits are the source for most human cases in endemic areas of the southeastern United States. In Utah, Nevada, and California, tabanid flies are the most common vectors. Animal reservoirs include wild rabbits, squirrels, birds, sheep, beavers, muskrats, and domestic dogs and cats. Person-to-person transmission is rare or nonexistent.
The four subspecies of F. tularensis are tularensis, holarctica, novicida, and mediasiatica. The first three of these subspecies are found in North America; in fact, subspecies tularensis has been isolated only in North America, where it accounts for >70% of cases of tularemia and produces more serious human disease than other subspecies (although, with treatment, the associated fatality rate is <2%). The progression of illness depends on the infecting strain’s virulence, the inoculum size, the portal of entry, and the host’s immune status.
Ticks pass F. tularensis to their offspring transovarially. The organism is found in tick feces but not in large quantities in tick salivary glands. In the United States, the disease is carried by Dermacentor andersoni (Rocky Mountain wood tick), Dermacentor variabilis (American dog tick), Dermacentor occidentalis (Pacific Coast dog tick), and Amblyomma americanum (Lone Star tick). F. tularensis is transmitted frequently during blood meals taken by embedded ticks after hours of attachment. It is the taking of a blood meal through a fecally contaminated field that transmits the organism. Transmission by ticks and tabanid flies takes place mainly in the spring and summer. However, continued transmission in the winter by trapped or hunted animals has been documented.
![]() Tularemia is most common in the southeastern United States; Arkansas, Missouri, and Oklahoma account for more than half of all reported cases in this country. Small outbreaks in higher-risk populations (e.g., professional landscapers cutting up brush, mowing, and using a leaf blower) have been reported. Although the irregular distribution of cases of tularemia makes worldwide estimates difficult, increasing numbers of cases have been reported between latitudes 30° and 71°N (the Holarctic region) in the Northern Hemisphere. Cases of tularemia have been reported from Europe, Turkey, Canada, Mexico, and Asia. If the disease is caused by subspecies tularensis, the clinical manifestations are similar to those in the United States. However, in areas where tularemia is due largely to subspecies holarctica, oropharyngeal disease is common. Disease acquisition results from the consumption of water contaminated by live organisms shed by animals (e.g., muskrats, beavers). Subspecies holarctica is known to cause milder disease than other subspecies and responds well to fluoroquinolones, especially ciprofloxacin.
Tularemia is most common in the southeastern United States; Arkansas, Missouri, and Oklahoma account for more than half of all reported cases in this country. Small outbreaks in higher-risk populations (e.g., professional landscapers cutting up brush, mowing, and using a leaf blower) have been reported. Although the irregular distribution of cases of tularemia makes worldwide estimates difficult, increasing numbers of cases have been reported between latitudes 30° and 71°N (the Holarctic region) in the Northern Hemisphere. Cases of tularemia have been reported from Europe, Turkey, Canada, Mexico, and Asia. If the disease is caused by subspecies tularensis, the clinical manifestations are similar to those in the United States. However, in areas where tularemia is due largely to subspecies holarctica, oropharyngeal disease is common. Disease acquisition results from the consumption of water contaminated by live organisms shed by animals (e.g., muskrats, beavers). Subspecies holarctica is known to cause milder disease than other subspecies and responds well to fluoroquinolones, especially ciprofloxacin.
PATHOGENESIS AND PATHOLOGY
The most common portal of entry for human infection is through skin or mucous membranes, either directly—through the bite of ticks, other arthropods, or other animals—or via inapparent abrasions. Inhalation or ingestion of F. tularensis also can result in infection. F. tularensis is extremely infectious: Although >108 organisms are usually required to produce infection via the oral route (oropharyngeal or gastrointestinal tularemia), as few as 10 organisms can result in infection when injected into the skin (ulceroglandular/glandular tularemia) or inhaled (pulmonary tularemia). After inoculation into the skin, the organism multiplies locally; within 2–5 days (range, 1–10 days), it produces an erythematous, tender, or pruritic papule. The papule rapidly enlarges and forms an ulcer with a black base (chancriform lesion). The bacteria spread to regional lymph nodes, producing lymphadenopathy (buboes). All forms can lead to bacteremia with spread to distant organs, including the central nervous system.
Tularemia is characterized by mononuclear cell infiltration with pyogranulomatous pathology. The histopathologic findings can be quite similar to those in tuberculosis, although tularemia develops more rapidly. As a facultatively intracellular bacterium, F. tularensis can parasitize both phagocytic and nonphagocytic host cells and can survive intracellularly for prolonged periods. In the acute phase of infection, the primary organs affected (skin, lymph nodes, liver, and spleen) include areas of focal necrosis, which are initially surrounded by polymorphonuclear leukocytes (PMNs). Subsequently, granulomas form, with epithelioid cells, lymphocytes, and multinucleated giant cells surrounded by areas of necrosis. These areas may resemble caseation necrosis but later coalesce to form abscesses.
Conjunctival inoculation can result in infection of the eye, with regional lymph node enlargement (preauricular lymphadenopathy, Parinaud’s complex). Aerosolization and inhalation or hematogenous spread of organisms can result in pneumonia. In the lung, an inflammatory reaction develops, including foci of alveolar necrosis and cell infiltration (initially polymorphonuclear and later mononuclear) with granulomas. Chest roentgenograms usually reveal bilateral patchy infiltrates rather than large areas of consolidation. Pleural effusions are common and may contain blood. Lymphadenopathy occurs in regions draining infected organs. Therefore, in pulmonary infection, mediastinal adenopathy may be evident, whereas patients with oropharyngeal tularemia develop cervical lymphadenopathy. In gastrointestinal or typhoidal tularemia, mesenteric lymphadenopathy may follow the ingestion of large numbers of organisms. (The term typhoidal tularemia may be used to describe severe bacteremic disease, irrespective of the mode of transmission or portal of entry.) Meningitis has been reported as a primary or secondary manifestation of bacteremia. Patients may also present with fever and no localizing signs.
IMMUNOLOGY
Although a complete and widely accepted understanding of the protective immune response to F. tularensis is lacking, significant advances in the study of natural and protective immunity have been made in recent years and may ultimately result in a vaccine candidate. Complete genomic sequencing and the availability of attenuated F. tularensis strains developed through genetic manipulation are facilitating research that will expand our knowledge in this area.
A number of investigators have studied various models and proposed various hypotheses regarding the induction of protective immunity to F. tularensis. Although further research is needed, synergy between humoral and cell-mediated immune (CMI) responses appears to be critical in inducing effective immune protection. Elucidation of the molecular mechanisms for the organism’s evasion of the host response, pathogen-associated molecular patterns, and effective host immune protection has led to novel vaccination strategies tested in animal models. Antibodies to Fc receptors on antigen-presenting cells have been shown to be protective in animal models of pulmonary tularemia, resulting in both mucosal and CMI responses. This enhanced understanding of mucosal and serum antibodies in combination with a targeted CMI response holds great promise for future vaccine development.
CLINICAL MANIFESTATIONS
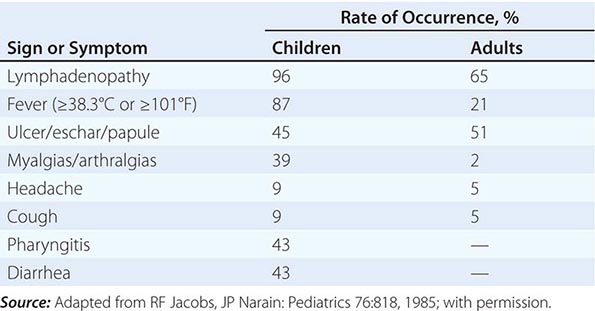
Tularemia often starts with a sudden onset of fever, chills, headache, and generalized myalgias and arthralgias (Table 195-1). This onset takes place when the organism penetrates the skin, is ingested, or is inhaled. An incubation period of 2–10 days is followed by the formation of an ulcer at the site of penetration, with local inflammation. The ulcer may persist for several months as organisms are transported via the lymphatics to the regional lymph nodes. These nodes enlarge and may become necrotic and suppurative. If the organism enters the bloodstream, widespread dissemination can result.
CLINICAL PRESENTATION OF TULAREMIA |
In the United States, most patients with tularemia (75–85%) acquire the infection by inoculation of the skin. In adults, the most common localized form is inguinal/femoral lymphadenopathy; in children, it is cervical lymphadenopathy. About 20% of patients develop a generalized maculopapular rash, which occasionally becomes pustular. Erythema nodosum occurs infrequently. The clinical manifestations of tularemia have been divided into various syndromes, which are listed in Table 195-2.
CLINICAL SYNDROMES OF TULAREMIA |
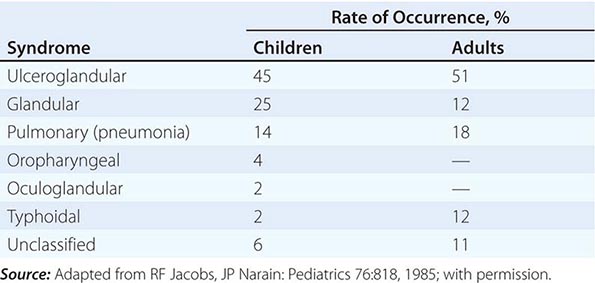
Ulceroglandular/Glandular Tularemia These two forms of tularemia account for ~75–85% of cases. The predominant form in children involves cervical or posterior auricular lymphadenopathy and is usually related to tick bites on the head and neck. In adults, the most common form is inguinal/femoral lymphadenopathy resulting from insect and tick exposures on the lower limbs. In cases related to wild game, the usual portal of entry for F. tularensis is either an injury sustained while skinning or cleaning an animal carcass or a bite (usually on the hand). Epitrochlear lymphadenopathy/lymphadenitis is common in patients with bite-related injuries.
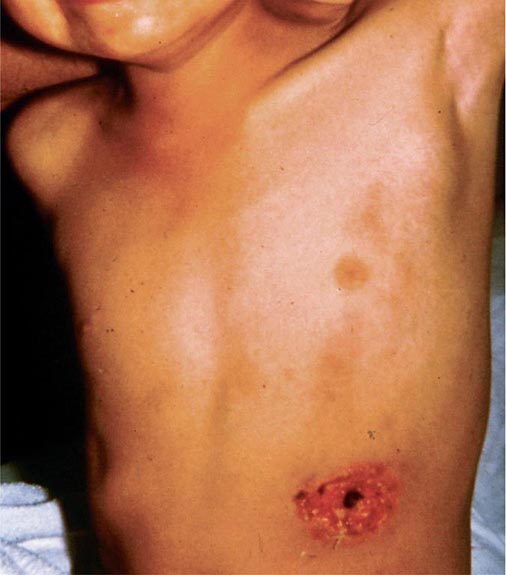
In ulceroglandular tularemia, the ulcer is erythematous, indurated, and nonhealing, with a punched-out appearance that lasts 1–3 weeks. The papule may begin as an erythematous lesion that is tender or pruritic; it evolves over several days into an ulcer with sharply demarcated edges and a yellow exudate. The ulcer gradually develops a black base, and simultaneously the regional lymph nodes become tender and severely enlarged (Fig. 195-1). The affected lymph nodes may become fluctuant and drain spontaneously, but the condition usually resolves with effective treatment. Late suppuration of lymph nodes has been described in up to 25% of patients with ulceroglandular/glandular tularemia. Examination of material taken from these late fluctuant nodes after successful antimicrobial treatment reveals sterile necrotic tissue. In 5–10% of patients, the skin lesion may be inapparent, with lymphadenopathy plus systemic signs and symptoms the only physical findings (glandular tularemia). Conversely, a tick or deerfly bite on the trunk may result in an ulcer without evident lymphadenopathy.
FIGURE 195-1 An 8-year-old boy with inguinal lymphadenitis and associated tick-bite site characteristic of ulceroglandular tularemia.
Oculoglandular Tularemia In ~1% of patients, the portal of entry for F. tularensis is the conjunctiva, which the organism usually reaches through contact with contaminated fingers. The inflamed conjunctiva is painful, with numerous yellowish nodules and pinpoint ulcers. Purulent conjunctivitis with regional lymphadenopathy (preauricular, submandibular, or cervical) is evident. Because of debilitating pain, the patient may seek medical attention before regional lymphadenopathy develops. Painful preauricular lymphadenopathy is unique to tularemia and distinguishes it from tuberculosis, sporotrichosis, and syphilis. Corneal perforation may occur.
Oropharyngeal and Gastrointestinal Tularemia Rarely, tularemia follows ingestion of contaminated undercooked meat, oral inoculation of F. tularensis from the hands in association with the skinning and cleaning of animal carcasses, or consumption of contaminated food or water. Oral inoculation may result in acute, exudative, or membranous pharyngitis associated with cervical lymphadenopathy or in ulcerative intestinal lesions associated with mesenteric lymphadenopathy, diarrhea, abdominal pain, nausea, vomiting, and gastrointestinal bleeding. Infected tonsils become enlarged and develop a yellowish-white pseudomembrane, which can be confused with that of diphtheria. The clinical severity of gastrointestinal tularemia varies from mild, unexplained, persistent diarrhea with no other symptoms to a fulminant, fatal disease. In fatal cases, the extensive intestinal ulceration found at autopsy suggests an enormous inoculum.
Pulmonary Tularemia Pneumonia due to F. tularensis presents as variable parenchymal infiltrates that are unresponsive to treatment with β-lactam antibiotics. Tularemia must be considered in the differential diagnosis of atypical pneumonia in a patient with a history of travel to an endemic area. The disease can result from inhalation of an infectious aerosol or can spread to the lungs and pleura via bacteremia. Inhalation-related pneumonia has been described in laboratory workers after exposure to contaminated materials and, if untreated, can be associated with a relatively high mortality rate. Exposure to F. tularensis in aerosols from live domestic animals or dead wildlife (including birds) has been reported to cause pneumonia. Hematogenous dissemination to the lungs occurs in 10–15% of cases of ulceroglandular tularemia and in about half of cases of typhoidal tularemia. Previously, tularemia pneumonia was thought to be a disease of older patients, but as many as 10–15% of children with clinical manifestations of tularemia have parenchymal infiltrates detected by chest roentgenography. Patients with pneumonia usually have a nonproductive cough and may have dyspnea or pleuritic chest pain. Roentgenograms of the chest usually reveal bilateral patchy infiltrates (described as ovoid or lobar densities), lobar parenchymal infiltrates, and cavitary lesions. Pleural effusions may have a predominance of mononuclear leukocytes or PMNs and sometimes red blood cells. Empyema may develop. Blood cultures may be positive for F. tularensis.
Typhoidal Tularemia The typhoidal presentation is now considered rare in the United States. The source of infection in typhoidal tularemia is usually associated with pharyngeal and/or gastrointestinal inoculation or bacteremic disease. Fever usually develops without apparent skin lesions or lymphadenopathy. Some patients have cervical and mesenteric lymphadenopathy. In the absence of a history of possible contact with a vector, diagnosis can be extremely difficult. Blood cultures may be positive and patients may present with classic sepsis or septic shock in this acute systemic form of the infection. Typhoidal tularemia is usually associated with a huge inoculum or with a preexisting compromising condition. High continuous fevers, signs of sepsis, and severe headache are common. The patient may be delirious and may develop prostration and shock. If presumptive antibiotic therapy in culture-negative cases does not include an aminoglycoside, the estimated mortality rate is relatively high.
Other Manifestations F. tularensis infection has been associated with meningitis, pericarditis, hepatitis, peritonitis, endocarditis, osteomyelitis, and sepsis and septic shock with rhabdomyolysis and acute renal failure. In cases of tularemia meningitis, a mean white blood cell count of 1788/μL, a predominantly mononuclear cell response (70–100%), a depressed glucose level, an elevated protein concentration, and a negative Gram’s stain are typically found on examination of cerebrospinal fluid.
DIFFERENTIAL DIAGNOSIS
When patients in endemic areas present with fever, chronic ulcerative skin lesions, and large tender lymph nodes (Fig. 195-1), a diagnosis of tularemia should be made presumptively, and confirmatory diagnostic testing and appropriate therapy should be undertaken. When the possibility of tularemia is considered in a nonendemic area, an attempt should be made to identify contact with a potential animal vector. The level of suspicion should be especially high in hunters, trappers, game wardens, professional landscapers, veterinarians, laboratory workers, and individuals exposed to an insect or another animal vector. However, up to 40% of patients with tularemia have no known history of epidemiologic contact with an animal vector.
The characteristic presentation of ulceroglandular tularemia does not pose a diagnostic problem, but a less classic progression of regional lymphadenopathy or glandular tularemia must be differentiated from other diseases (Table 195-3). The skin lesion of tularemia may resemble those seen in various other diseases but is generally accompanied by more impressive regional lymphadenopathy. In children, the differentiation of tularemia from cat-scratch disease is made more difficult by the chronic papulovesicular lesion associated with Bartonella henselae infection (Chap. 197). Oropharyngeal tularemia can resemble and must be differentiated from pharyngitis due to other bacteria or viruses. Pulmonary tularemia may resemble any atypical pneumonia. Typhoidal tularemia and tularemia meningitis may resemble a variety of other infections.
TULAREMIA: DIFFERENTIAL DIAGNOSIS, BY CLINICAL DISEASE CATEGORY |
LABORATORY DIAGNOSIS
The diagnosis of tularemia is most frequently confirmed by agglutination testing. Microagglutination and tube agglutination are the techniques most commonly used to detect antibody to F. tularensis. In the standard tube agglutination test, a single titer of ≥1:160 is interpreted as a presumptive positive result. A fourfold increase in titer between paired serum samples collected 2–3 weeks apart is considered diagnostic. False-negative serologic responses are obtained early in infection; up to 30% of patients infected for 3 weeks have sera that test negative. Late in infection, titers into the thousands are common, and titers of 1:20–1:80 may persist for years. Enzyme-linked immunosorbent assays have proved useful for the detection of both antibodies and antigens.
Culture and isolation of F. tularensis are difficult. In one study, the organism was isolated in only 10% of more than 1000 human cases, 84% of which were confirmed by serology. The medium of choice is cysteine-glucose-blood agar. F. tularensis can be isolated directly from infected ulcer scrapings, lymph node biopsy specimens, gastric washings, sputum, and blood cultures. Colonies are blue-gray, round, smooth, and slightly mucoid. On media containing blood, a small zone of α hemolysis usually surrounds the colony. Slide agglutination tests or direct fluorescent antibody tests with commercially available antisera can be applied directly to culture suspensions for identification. Most clinical laboratories will not attempt to culture F. tularensis because of the infectivity of the organism from the culture media and the consequent risk of a laboratory-acquired infection. Although tularemia is not spread from person to person, the organism can be inhaled from culture plates and infect unsuspecting laboratory workers. In most clinical laboratories, biosafety level 2 practices are recommended to handle clinical specimens thought to contain F. tularensis; however, biosafety level 3 conditions are required for procedures that produce aerosols or droplets during manipulation of cultures containing or possibly containing this organism.
A variety of polymerase chain reaction (PCR) methods have been used to detect F. tularensis DNA in many clinical specimens but mostly in ulceroglandular disease. The majority of these methods target the genes encoding outer-membrane proteins (e.g., fopA or tul4). A 16S rDNA sequence identification PCR may be helpful when the patient’s clinical information does not lead the clinician to suspect a diagnosis of tularemia.
TREATMENT | TULAREMIA |
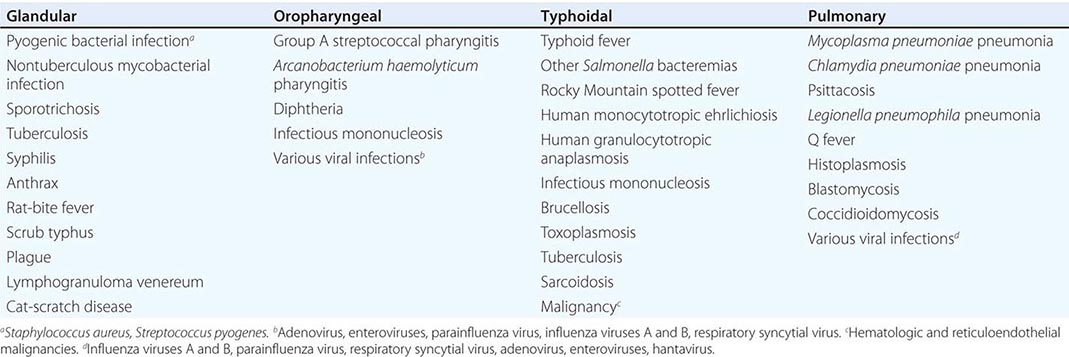
Only aminoglycosides, tetracyclines, chloramphenicol, and rifampin are currently approved by the U.S. Food and Drug Administration for the treatment of tularemia. Gentamicin is considered the drug of choice for both adults and children. The dosage for adults and children is 5 mg/kg daily in two divided doses. Gentamicin therapy is typically continued for 7–10 days; however, in mild to moderate cases of tularemia in which the patient becomes afebrile within the first 48–72 h of gentamicin treatment, a 5- to 7-day course has been successful.
If available, streptomycin given intramuscularly is also effective. The dosage for adults is 2 g/d in two divided doses. For children, the dosage is 30 mg/kg daily in two divided doses (maximal daily dose, 2 g). After a clinical response is demonstrated at 3–5 days, the dosage for children can be reduced to 10–15 mg/kg daily in two divided doses. The total duration of streptomycin therapy in both adults and children is usually 10 days. Unlike streptomycin and gentamicin, tobramycin is ineffective in the treatment of tularemia and should not be used.
Because doxycycline is bacteriostatic against F. tularensis, there is a risk of relapse if the patient is not treated for a long enough period. Therefore, if doxycycline is used, it should be given for at least 14 days. The lack of availability of chloramphenicol limits the utility of this agent as a viable treatment option. Fluoroquinolones—specifically, ciprofloxacin and levofloxacin—have been used with good outcomes to treat infections caused by subspecies holarctica, which is most often found in Europe. The lack of data on the efficacy of these agents against subspecies tularensis limits their use in North America at this time.
F. tularensis cannot be subjected to standardized antimicrobial susceptibility testing because the organism will not grow on the media used. A wide variety of antibiotics, including all β-lactam antibiotics and the cephalosporins, are ineffective for the treatment of tularemia. Several studies indicated that third-generation cephalosporins were active against F. tularensis in vitro, but clinical case reports suggested nearly universal failure of ceftriaxone in pediatric patients with tularemia. Although in vitro data indicate that imipenem may be active, therapy with imipenem, sulfanilamides, and macrolides is not presently recommended because of the lack of relevant clinical data.
Virtually all strains of F. tularensis are susceptible to streptomycin and gentamicin. Hearing screening should be considered before initiation of streptomycin or gentamicin therapy. In successfully treated patients, defervescence usually occurs within 2 days, but skin lesions and lymph nodes may take 1–2 weeks to heal. When therapy is not initiated within the first several days of illness, defervescence may be delayed. Relapses are uncommon with streptomycin or gentamicin therapy. Late lymph-node suppuration, however, occurs in ~40% of children, regardless of the treatment received. These nodes have typically been found to contain sterile necrotic tissue without evidence of active infection. Patients with fluctuant nodes should receive several days of antibiotic therapy before drainage to minimize the risk to hospital personnel.
PROGNOSIS
If tularemia goes untreated, symptoms usually last 1–4 weeks but may continue for months. The mortality rate from severe untreated infection (including all cases of untreated pulmonary and typhoidal tularemia) can be as high as 30%. However, the overall mortality rate for untreated tularemia is <8%. With appropriate treatment, the mortality rate is <1%. Poor outcomes are often associated with long delays in diagnosis and treatment. Lifelong immunity usually follows tularemia.
PREVENTION
The prevention of tularemia is based on avoidance of exposure to biting and blood-sucking insects, especially ticks and deerflies. A wide range of approaches to vaccine development are being evaluated, but no vaccine against tularemia is yet licensed. Prophylaxis of tularemia has not proved effective in patients with embedded ticks or insect bites. However, in patients who are known to have been exposed to large quantities of organisms (e.g., in the laboratory) and who have incubating infection with F. tularensis, early treatment can prevent the development of significant clinical disease.
196 | Plague and Other Yersinia Infections |
PLAGUE
Plague is a systemic zoonosis caused by Yersinia pestis. It predominantly affects small rodents in rural areas of Africa, Asia, and the Americas and is usually transmitted to humans by an arthropod vector (the flea). Less often, infection follows contact with animal tissues or respiratory droplets. Plague is an acute febrile illness that is treatable with antimicrobial agents, but mortality rates among untreated patients are high. Ancient DNA studies have confirmed that the fourteenth-century “Black Death” in Europe was Y. pestis infection. Patients can present with the bubonic, septicemic, or pneumonic form of the disease. Although there is concern among the general public about epidemic spread of plague by the respiratory route, this is not the usual route of plague transmission, and established infection-control measures for respiratory plague exist. However, the fatalities associated with plague and the capacity for infection via the respiratory tract mean that Y. pestis fits the profile of a potential agent of bioterrorism. Consequently, measures have been taken to restrict access to the organism, including legislation affecting diagnostic and research procedures in some countries (e.g., the United States).
ETIOLOGY
The genus Yersinia comprises gram-negative bacteria of the family Enterobacteriaceae (gamma proteobacteria). Overwhelming taxonomic evidence showing Y. pestis strains as a clonal group within Yersinia pseudotuberculosis suggests recent evolution from the latter organism—an enteric pathogen of mammals that is spread by the fecal-oral route and thus has a phenotype distinctly different from that of Y. pestis. When grown in vivo or at 37°C, Y. pestis forms an amorphous capsule made from a plasmid-specified fimbrial protein, Caf or fraction 1 (F1) antigen, which is an immunodiagnostic marker of infection.
EPIDEMIOLOGY
Human plague generally follows an outbreak in a host rodent population (epizootic). Mass deaths among the rodent primary hosts lead to a search by fleas for new hosts, with consequent incidental infection of other mammals. The precipitating cause for an epizootic may ultimately be related to climate or other environmental factors. The reservoir for Y. pestis causing enzootic plague in natural endemic foci between epizootics (i.e., when the organism may be difficult to detect in rodents or fleas) is a topic of ongoing research and may not be the same in all regions. The enzootic/epizootic pattern may be the result of complex dynamic interactions of host rodents that have different plague susceptibilities and different flea vectors; alternatively, an environmental reservoir may be important.
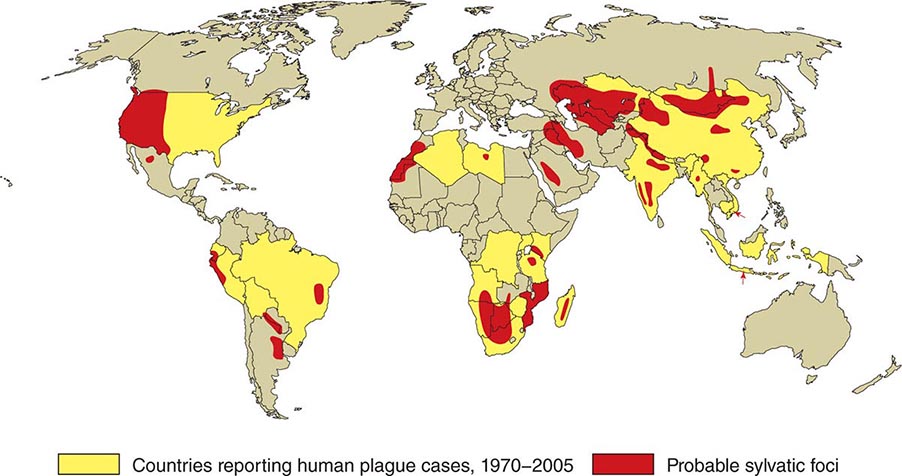
![]() In general, the enzootic areas for plague are lightly populated regions of Africa, Asia, and the Americas (Fig. 196-1). Between 2004 and 2009, 12,503 cases of plague, with a global case-fatality rate of 6.7%, were recorded by the World Health Organization (WHO); these figures were obtained by combining cases notified under the International Health Regulations with data from national surveillance programs and publications. More than 97% of these cases were in Africa; the majority of cases were reported from the Democratic Republic of the Congo and the island of Madagascar. The period covered spans a change in the International Health Regulations from a requirement for nations to notify the WHO of all cases of plague to a requirement to report pneumonic plague or any suspected case of plague in an area not known to be endemic for plague. In the past decade, outbreaks of pneumonic plague have been recorded in the Democratic Republic of the Congo, Uganda, Algeria, Madagascar, China, and Peru.
In general, the enzootic areas for plague are lightly populated regions of Africa, Asia, and the Americas (Fig. 196-1). Between 2004 and 2009, 12,503 cases of plague, with a global case-fatality rate of 6.7%, were recorded by the World Health Organization (WHO); these figures were obtained by combining cases notified under the International Health Regulations with data from national surveillance programs and publications. More than 97% of these cases were in Africa; the majority of cases were reported from the Democratic Republic of the Congo and the island of Madagascar. The period covered spans a change in the International Health Regulations from a requirement for nations to notify the WHO of all cases of plague to a requirement to report pneumonic plague or any suspected case of plague in an area not known to be endemic for plague. In the past decade, outbreaks of pneumonic plague have been recorded in the Democratic Republic of the Congo, Uganda, Algeria, Madagascar, China, and Peru.
FIGURE 196-1 Approximate global distribution of Yersinia pestis. (Compiled from WHO, CDC, and country sources. Reprinted with permission from DT Dennis, GL Campbell: Plague and other Yersinia infections, in Harrison’s Principles of Internal Medicine, 17th ed, AS Fauci et al [eds]. New York, McGraw-Hill, Chap. 152, 2008.)
Plague was introduced into North America via the port of San Francisco in 1900 as part of the Third Pandemic, which spread around the world from Hong Kong. The disease is presently enzootic on the western side of the continent from southwestern Canada to Mexico. Most human cases in the United States occur in two regions: “Four Corners” (the junction point of New Mexico, Arizona, Colorado, and Utah), especially northern New Mexico, northern Arizona, and southern Colorado; and further west in California, southern Oregon, and western Nevada (http://www.cdc.gov/plague/maps/index.html). From 1990 to 2011, 151 cases of plague were reported in the United States, a mean of seven cases per year. Most cases occurred from May to October—the time of year when people are outdoors and rodents and their fleas are most plentiful. The infection is most often acquired by fleabite in peridomestic environments; it can also be acquired through the handling of living or dead small mammals (e.g., rabbits, hares, and prairie dogs) or wild carnivores (e.g., wildcats, coyotes, or mountain lions). Dogs and cats may bring plague-infected fleas into the home, and infected cats may transmit plague directly to humans by the respiratory route. The last recorded case of person-to-person transmission in the United States occurred in 1925.
Plague most often develops in areas with poor sanitary conditions and infestations of rats—in particular, the widely distributed roof rat Rattus rattus and the brown rat Rattus norvegicus (which serves as a laboratory model of plague). Rat control in warehouses and shipping facilities has been recognized as important in preventing the spread of plague since the early twentieth century and features in the current WHO International Health Regulations. Urban rodents acquire infection from wild rodents, and the proximity of the former to humans increases the risk of transmission. The oriental rat flea Xenopsylla cheopis is the most efficient vector for transmission of plague among rats and onward to humans in Asia, Africa, and South America.
Worldwide, bubonic plague is the predominant form reported (80–95% of suspected cases), with mortality rates of 10–20%. The mortality rate is higher (22%) in the small proportion of patients (10–20%) with primary septicemic plague (i.e., systemic Y. pestis sepsis with no bubo; see “Clinical Manifestations,” below) and is highest with primary pulmonary plague; in this, the least common of the main plague presentations, the mortality rate approaches 100% without antimicrobial treatment and is >50% even with such treatment. Rare outbreaks of pharyngeal plague following consumption of raw or undercooked camel or goat meat have been reported.
A total of 81 (76%) of the 107 plague cases reported in the United States from 1990 to 2005 were primary bubonic disease, 19 (18%) were primary septicemic disease, and 5 (5%) were primary pneumonic disease; 2 cases (2%) were not classified. Eleven cases (10%) were fatal.
PATHOGENESIS
![]() As mentioned earlier, genetic evidence suggests that Y. pestis is a clone derived from the enteric pathogen Y. pseudotuberculosis in the recent evolutionary past (9000–40,000 years ago). The change from infection by the fecal-oral route to a two-stage life cycle, with alternate parasitization of arthropod and mammalian hosts, followed the acquisition of two plasmids (pFra and pPst) and the inactivation of remarkably few Y. pseudotuberculosis genes in conjunction with preexisting properties of the Y. pseudotuberculosis ancestor (e.g., the presence of a third plasmid, pYV, and the capacity to cause septicemia). In the arthropod-parasitizing portion of its life cycle, Y. pestis multiplies and forms biofilm-embedded aggregates in the flea midgut after ingestion of a blood meal containing bacteria. In some fleas, biofilm-embedded bacteria eventually fill the proventriculus (a valve connecting the esophagus to the midgut) and block normal blood feeding. Both “blocked” fleas and those containing masses of biofilm-embedded Y. pestis without complete blockage inoculate Y. pestis into each bite site. The ability of Y. pestis to colonize and multiply in the flea requires phospholipase D encoded by the ymt gene on the pFra plasmid, and biofilm synthesis requires the chromosomal hms locus shared with Y. pseudotuberculosis. However, three Y. pseudotuberculosis genes inhibiting biofilm formation or promoting its degradation are inactivated in Y. pestis, together with urease, which causes acute flea gastrointestinal toxicity. Blockage takes days or weeks to come about after initial infection of the flea and is followed by the flea’s death. In addition, many flea vectors (including X. cheopis) are able to transmit plague in an early-phase unblocked state for up to 1 week after feeding, but 10 fleas in this state are required to infect a mammalian host (mass transmission).
As mentioned earlier, genetic evidence suggests that Y. pestis is a clone derived from the enteric pathogen Y. pseudotuberculosis in the recent evolutionary past (9000–40,000 years ago). The change from infection by the fecal-oral route to a two-stage life cycle, with alternate parasitization of arthropod and mammalian hosts, followed the acquisition of two plasmids (pFra and pPst) and the inactivation of remarkably few Y. pseudotuberculosis genes in conjunction with preexisting properties of the Y. pseudotuberculosis ancestor (e.g., the presence of a third plasmid, pYV, and the capacity to cause septicemia). In the arthropod-parasitizing portion of its life cycle, Y. pestis multiplies and forms biofilm-embedded aggregates in the flea midgut after ingestion of a blood meal containing bacteria. In some fleas, biofilm-embedded bacteria eventually fill the proventriculus (a valve connecting the esophagus to the midgut) and block normal blood feeding. Both “blocked” fleas and those containing masses of biofilm-embedded Y. pestis without complete blockage inoculate Y. pestis into each bite site. The ability of Y. pestis to colonize and multiply in the flea requires phospholipase D encoded by the ymt gene on the pFra plasmid, and biofilm synthesis requires the chromosomal hms locus shared with Y. pseudotuberculosis. However, three Y. pseudotuberculosis genes inhibiting biofilm formation or promoting its degradation are inactivated in Y. pestis, together with urease, which causes acute flea gastrointestinal toxicity. Blockage takes days or weeks to come about after initial infection of the flea and is followed by the flea’s death. In addition, many flea vectors (including X. cheopis) are able to transmit plague in an early-phase unblocked state for up to 1 week after feeding, but 10 fleas in this state are required to infect a mammalian host (mass transmission).
Y. pestis disseminates from the site of inoculation in the mammalian host in a process initially dependent on plasminogen activator Pla, which is encoded by the small pPst plasmid. This surface protease activates mammalian plasminogen, degrades complement, and adheres to the extracellular matrix component laminin. Pla is essential for the high-level virulence of Y. pestis in mice by subcutaneous or intradermal injection (laboratory proxies for fleabites) and for the development of primary pneumonic plague. When actual fleabite inoculation is used in mouse models, the fimbrial capsule-forming protein (Ca1 or fraction 1; F1 antigen) encoded on pFra increases the efficiency of transmission, and plasminogen activator is required for the formation of buboes. Because the antiphagocytic systems in Y. pestis are not fully operational at the time of inoculation into the mammalian host, the organism is taken up by macrophages from the inoculation site and transported to regional lymph nodes. After intracellular replication, Y. pestis switches to extracellular replication with full expression of its antiphagocytic systems: the type III secretion machines and their effectors encoded by pYV as well as the F1 capsule. Overproduction of the type III secretion substrate and translocation protein LcrV exerts an anti-inflammatory effect, reducing host immune responses. Likewise, Y. pestis lipopolysaccharide is modified to minimize stimulation of host Toll-like receptor 4, thereby reducing protective host inflammatory responses during peripheral infection and prolonging host survival with high-grade bacteremia—an effect that probably enhances the pathogen’s subsequent transmission by fleabite.
Replication of Y. pestis in a regional lymph node results in the local swelling of the lymph node and periglandular region known as a bubo. On histology, the node is found to be hemorrhagic or necrotic, with thrombosed blood vessels, and the lymphoid cells and normal architecture are replaced by large numbers of bacteria and fibrin. Periglandular tissues are inflamed and also contain large numbers of bacteria in a serosanguineous, gelatinous exudate.
Continued spread through the lymphatic vessels to contiguous lymph nodes produces second-order primary buboes. Infection is initially contained in the infected regional lymph nodes, although transient bacteremia can be detected. As the infection progresses, spread via efferent lymphatics to the thoracic duct produces high-grade bacteremia. Hematogenous spread to the spleen, liver, and secondary buboes follows, with subsequent uncontrolled septicemia, endotoxic shock, and disseminated intravascular coagulation leading to death. In some patients, this septicemic phase occurs without obvious prior bubo development or lung disease (septicemic plague). Hematogenous spread to the lungs results in secondary plague pneumonia, with bacteria initially more prominent in the interstitium than in the air spaces (the reverse being the case in primary plague pneumonia). Hematogenous spread to other organs, including the meninges, can occur.
CLINICAL MANIFESTATIONS
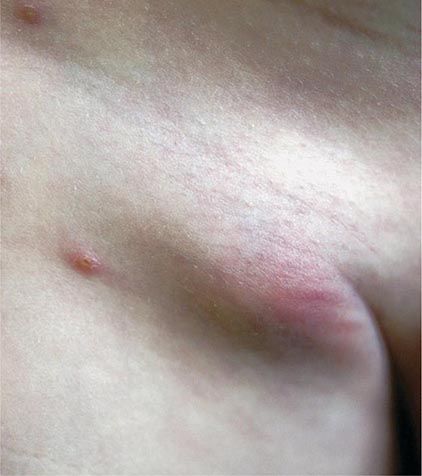
Bubonic Plague After an incubation period of 2–6 days, the onset of bubonic plague is sudden and is characterized by fever (>38°C), malaise, myalgia, dizziness, and increasing pain due to progressive lymphadenitis in the regional lymph nodes near the fleabite or other inoculation site. Lymphadenitis manifests as a tense, tender swelling (bubo) that, when palpated, has a boggy consistency with an underlying hard core. Generally, there is one painful and erythematous bubo with surrounding periganglionic edema. The bubo is most commonly inguinal but can also be crural, axillary (Fig. 196-2), cervical, or submaxillary, depending on the site of the bite. Abdominal pain from intraabdominal node involvement can occur without other visible signs. Children are most likely to present with cervical or axillary buboes.
FIGURE 196-2 Plague patient in the southwestern United States with a left axillary bubo and an unusual plague ulcer and eschar at the site of the infective flea bite. (Reprinted with permission from DT Dennis, GL Campbell: Plague and other Yersinia infections, in Harrison’s Principles of Internal Medicine, 17th ed, AS Fauci et al [eds]. New York, McGraw-Hill, Chap. 152, 2008.)
The differential diagnosis includes acute focal lymphadenopathy of other etiologies, such as streptococcal or staphylococcal infection, tularemia, cat-scratch disease, tick typhus, infectious mononucleosis, or lymphatic filariasis. These infections do not progress as rapidly, are not as painful, and are associated with visible cellulitis or ascending lymphangitis—both of which are absent in plague.
Without treatment, Y. pestis dissemination occurs and causes serious illness, including pneumonia (secondary pneumonic plague) and meningitis. Secondary pneumonic plague can be the source of person-to-person transmission of respiratory infection by productive cough (droplet infection), with the consequent development of primary plague pneumonia. Appropriate treatment of bubonic plague results in fever resolution within 2–5 days, but buboes may remain enlarged for >1 week after initial treatment and can become fluctuant.
Primary Septicemic Plague A minority (10–25%) of infections with Y. pestis present as gram-negative septicemia (hypotension, shock) without preceding lymphadenopathy. Septicemic plague occurs in all age groups, but persons older than age 40 years are at elevated risk. Some chronic conditions may predispose to septicemic plague: in 2009 in the United States, a fatal laboratory-acquired infection with an attenuated Y. pestis strain manifested as septicemic plague in a 60-year-old researcher with diabetes mellitus and undiagnosed hemochromatosis. These conditions also carry an increased risk of septicemia with other pathogenic Yersinia species. The term septicemic plague can be confusing since most patients with buboes have detectable bacteremia at some stage, with or without systemic signs of sepsis. In laboratory experiments, however, septicemic disease without histologic changes in lymph nodes is seen in a minority of mice infected via fleabites.
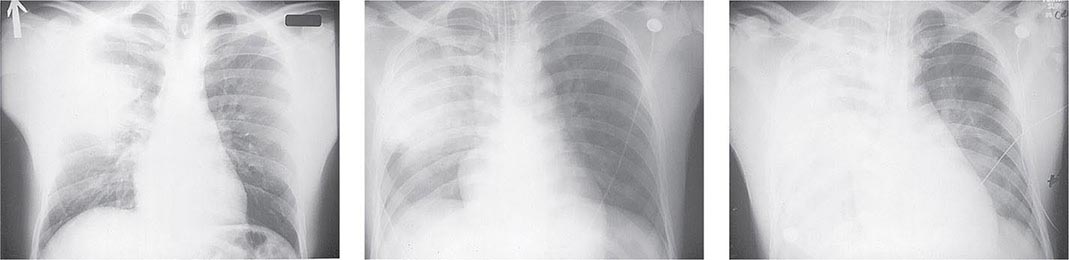
Pneumonic Plague Primary pneumonic plague results from inhalation of infectious bacteria in droplets expelled from another person or an animal with primary or secondary plague pneumonia. This syndrome has a short incubation period, averaging from a few hours to 2–3 days (range, 1–7 days), and is characterized by a sudden onset of fever, headache, myalgia, weakness, nausea, vomiting, and dizziness. Respiratory signs—cough, dyspnea, chest pain, and sputum production with hemoptysis—typically arise after 24 h. Progression of initial segmental pneumonitis to lobar pneumonia and then to bilateral lung involvement may occur (Fig. 196-3). The possible release of aerosolized Y. pestis bacteria in a bioterrorist attack, manifesting as an outbreak of primary pneumonic plague in nonendemic regions or in an urban setting where plague is rarely seen, has been a source of public health concern. Secondary pneumonic plague is a consequence of bacteremia occurring in ~10–15% of patients with bubonic plague. Bilateral alveolar infiltrates are seen on chest x-ray, and diffuse interstitial pneumonitis with scanty sputum production is typical.
FIGURE 196-3 Sequential chest radiographs of a patient with fatal primary plague pneumonia. Left: Upright posteroanterior film taken at admission to the hospital emergency department on the third day of illness, showing segmental consolidation of the right upper lobe. Center: Portable anteroposterior film taken 8 h after admission, showing extension of pneumonia to the right middle and right lower lobes. Right: Portable anteroposterior film taken 13 h after admission (when the patient had clinical adult respiratory distress syndrome), showing diffuse infiltration throughout the right lung and patchy infiltration of the left lower lung. A cavity later developed at the site of the initial right-upper-lobe consolidation. (Reprinted with permission from DT Dennis, GL Campbell: Plague and other Yersinia infections, in Harrison’s Principles of Internal Medicine, 17th ed., AS Fauci et al [eds]. New York, McGraw-Hill, Chap. 152, 2008.)
Meningitis Meningeal plague is uncommon, occurring in ≤6% of plague cases reported in the United States. Presentation with headache and fever typically occurs >1 week after the onset of bubonic or septicemic plague and may be associated with suboptimal antimicrobial therapy (delayed therapy, penicillin administration, or low-dose tetracycline treatment) and cervical or axillary buboes.
Pharyngitis Symptomatic plague pharyngitis can follow the consumption of contaminated meat from an animal dying of plague or contact with persons or animals with pneumonic plague. This condition can resemble tonsillitis, with peritonsillar abscess and cervical lymphadenopathy. Asymptomatic pharyngeal carriage of Y. pestis can also occur in close contacts of patients with pneumonic plague.
LABORATORY DIAGNOSIS
![]() Because of the scarcity of laboratory facilities in regions where human Y. pestis infection is most common, and because of the potential significance of Y. pestis isolation in a nonendemic area or an area from which human plague has been absent for many years, the WHO recommends an initial presumptive diagnosis followed by reference laboratory confirmation (Table 196-1). In the United States, comprehensive national diagnostic facilities for plague have been in place since a federal Laboratory Response Network (LRN; www.bt.cdc.gov/lrn/) was set up in 1999 to detect possible use of biological terrorism agents, including Y. pestis. Routine diagnostic clinical microbiology laboratories that are included in this network as sentinel-level laboratories use joint protocols from the Centers for Disease Control and Prevention (CDC) and the American Society for Microbiology to identify suspected Y. pestis isolates and to refer these specimens to LRN reference laboratories for confirmatory tests (http://www.asm.org/index.php/issues/sentinel-laboratory-guidelines). Y. pestis is designated a “Tier 1 select agent” under the Public Health Security and Bioterrorism Preparedness and Response Act of 2002 and subsequent executive orders; the provisions of this act, the Patriot Act of 2001, and related executive orders apply to all U.S. laboratories and individuals working with Y. pestis. Details of the applicable regulations are available from the CDC.
Because of the scarcity of laboratory facilities in regions where human Y. pestis infection is most common, and because of the potential significance of Y. pestis isolation in a nonendemic area or an area from which human plague has been absent for many years, the WHO recommends an initial presumptive diagnosis followed by reference laboratory confirmation (Table 196-1). In the United States, comprehensive national diagnostic facilities for plague have been in place since a federal Laboratory Response Network (LRN; www.bt.cdc.gov/lrn/) was set up in 1999 to detect possible use of biological terrorism agents, including Y. pestis. Routine diagnostic clinical microbiology laboratories that are included in this network as sentinel-level laboratories use joint protocols from the Centers for Disease Control and Prevention (CDC) and the American Society for Microbiology to identify suspected Y. pestis isolates and to refer these specimens to LRN reference laboratories for confirmatory tests (http://www.asm.org/index.php/issues/sentinel-laboratory-guidelines). Y. pestis is designated a “Tier 1 select agent” under the Public Health Security and Bioterrorism Preparedness and Response Act of 2002 and subsequent executive orders; the provisions of this act, the Patriot Act of 2001, and related executive orders apply to all U.S. laboratories and individuals working with Y. pestis. Details of the applicable regulations are available from the CDC.
WORLD HEALTH ORGANIZATION CASE DEFINITIONS OF PLAGUE |

Yersinia species are gram-negative coccobacilli (short rods with rounded ends) 1–3 μm in length and 0.5–0.8 μm in diameter. Y. pestis in particular appears bipolar (with a “closed safety pin” appearance) and pleomorphic when stained with a polychromatic stain (Wayson or Wright-Giemsa; Fig. 196-4). Its lack of motility distinguishes Y. pestis from other Yersinia species, which are motile at 25°C and nonmotile at 37°C. Transport medium (e.g., Cary-Blair medium) preserves the viability of Y. pestis if transport is delayed.
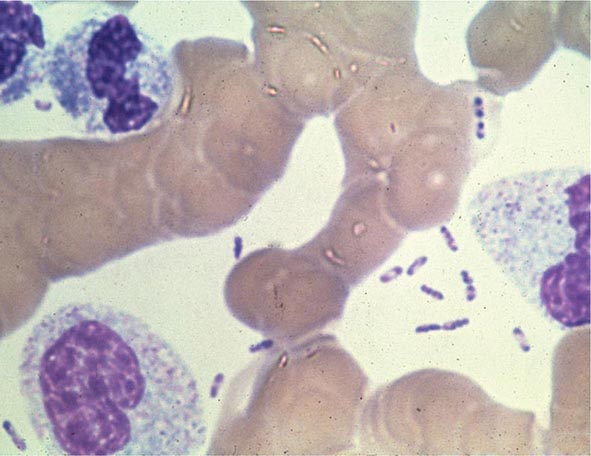
FIGURE 196-4 Peripheral blood smear from a patient with fatal plague septicemia and shock, showing characteristic bipolar-staining Yersinia pestis bacilli (Wright’s stain, oil immersion). (Reprinted with permission from DT Dennis, GL Campbell: Plague and other Yersinia infections, in Harrison’s Principles of Internal Medicine, 17th ed, AS Fauci et al [eds]. New York, McGraw-Hill, Chap. 152, 2008.)
The appropriate specimens for diagnosis of bubonic, pneumonic, and septicemic plague are bubo aspirate, bronchoalveolar lavage fluid or sputum, and blood, respectively. Culture of postmortem organ biopsy samples can also be diagnostic. A bubo aspirate is obtained by injection of 1 mL of sterile normal saline into a bubo under local anesthetic and aspiration of a small amount of (usually blood-stained) fluid. Gram’s staining of these specimens may reveal gram-negative rods, which are shown by Wayson or Wright-Giemsa staining to be bipolar. These bacteria may even be visible in direct blood smears in septicemic plague (Fig. 196-4); this finding indicates very high numbers of circulating bacteria and a poor prognosis.
Y. pestis grows on nutrient agar and other standard laboratory media but forms smaller colonies than do other Enterobacteriaceae. Specimens should be inoculated onto nutrient-rich media such as sheep blood agar (SBA), into nutrient-rich broth such as brain-heart infusion broth, and onto selective agar such as MacConkey or eosin methylene blue (EMB) agar. Yersinia-specific CIN (cefsulodin, triclosan [Irgasan], novobiocin) agar can be useful for culture of contaminated specimens, such as sputum. Blood should be cultured in a standard blood culture system. The optimal growth temperature is <37°C (25–29°C), with pinpoint colonies only on SBA at 24 h. Slower growth occurs at 37°C. Y. pestis is oxidase-negative, catalase-positive, urea-negative, indole-negative, and lactose-negative. Automated biochemical identification systems can misidentify Y. pestis as Y. pseudotuberculosis or other bacterial species.
![]() Reference laboratory tests for definitive identification of isolates include direct immunofluorescence for F1 antigen; specific polymerase chain reaction (PCR) for targets such as F1 antigen, the pesticin gene, and the plasminogen activator gene; and specific bacteriophage lysis. PCR can also be applied to diagnostic specimens, as can direct immunofluorescence for F1 antigen (produced in large amounts by Y. pestis) by slide microscopy. An immunochromatographic test strip for F1 antigen detection by monoclonal antibodies in clinical specimens has been devised in Madagascar. This method is effective for both laboratory and near-patient use and is now widely used in endemic countries. A similar test strip for Pla antigen has recently been developed and could be used to detect wild-type or engineered F1-negative virulent strains. Many other rapid diagnostic kits for possible bioterrorism pathogens, including Y. pestis, have been described in recent years, but none is widely used for primary or reference laboratory identification, and only one (a field real-time PCR for a range of potential bioterrorism agents) is approved by the U.S. Food and Drug Administration (FDA). Detailed phylogeographic DNA sequence data based on culture collections have been accumulated to trace plague evolution, and this system could be adapted in the future to determine real-time clinical plague epidemiology.
Reference laboratory tests for definitive identification of isolates include direct immunofluorescence for F1 antigen; specific polymerase chain reaction (PCR) for targets such as F1 antigen, the pesticin gene, and the plasminogen activator gene; and specific bacteriophage lysis. PCR can also be applied to diagnostic specimens, as can direct immunofluorescence for F1 antigen (produced in large amounts by Y. pestis) by slide microscopy. An immunochromatographic test strip for F1 antigen detection by monoclonal antibodies in clinical specimens has been devised in Madagascar. This method is effective for both laboratory and near-patient use and is now widely used in endemic countries. A similar test strip for Pla antigen has recently been developed and could be used to detect wild-type or engineered F1-negative virulent strains. Many other rapid diagnostic kits for possible bioterrorism pathogens, including Y. pestis, have been described in recent years, but none is widely used for primary or reference laboratory identification, and only one (a field real-time PCR for a range of potential bioterrorism agents) is approved by the U.S. Food and Drug Administration (FDA). Detailed phylogeographic DNA sequence data based on culture collections have been accumulated to trace plague evolution, and this system could be adapted in the future to determine real-time clinical plague epidemiology.
In the absence of other positive laboratory diagnostic tests, a retrospective serologic diagnosis may be made on the basis of rising titers of hemagglutinating antibody to F1 antigen. Enzyme-linked immunosorbent assays (ELISAs) for IgG and IgM antibodies to F1 antigen are also available.
The white blood cell (WBC) count is generally raised (to 10,000–20,000/μL) in plague, with neutrophilic leukocytosis and a left shift (numerous immature neutrophils); in some cases, however, the WBC count is normal or leukopenia develops. WBC counts are occasionally very high, especially in children (>100,000/μL). Levels of fibrinogen degradation products are elevated in a majority of patients, but platelet counts are usually normal or low-normal. However, disseminated intravascular coagulation, with low platelet counts, prolonged prothrombin times, reduced fibrinogen, and elevated fibrinogen degradation product levels, occurs in a significant minority of patients.
TREATMENT | PLAGUE |
Guidelines for the treatment of plague are given in Table 196-2. A 10-day course of antimicrobial therapy is recommended. Streptomycin has historically been the parenteral treatment of choice for plague and is approved for this indication by the FDA. Although not yet approved by the FDA for plague, gentamicin has proven safe and effective in clinical trials in Tanzania and Madagascar and in retrospectively reviewed cases in the United States. In view of streptomycin’s adverse-reaction profile and limited availability, some experts now recommend gentamicin over streptomycin. In 2012, the FDA approved levofloxacin for prophylaxis and treatment of plague (including septicemic and pneumonic disease), making it the first antibiotic approved for a new indication under a regulatory approach based on animal studies alone, known as the Animal Rule. An FDA decision on ciprofloxacin is pending. Levofloxacin is more efficacious than ciprofloxacin for postexposure prophylaxis of inhalational anthrax in animal models and also received FDA approval for this indication (Chap. 261e); thus it is approved for multiagent prophylaxis in possible bioterrorism exposures.
GUIDELINES FOR THE TREATMENT OF PLAGUE |
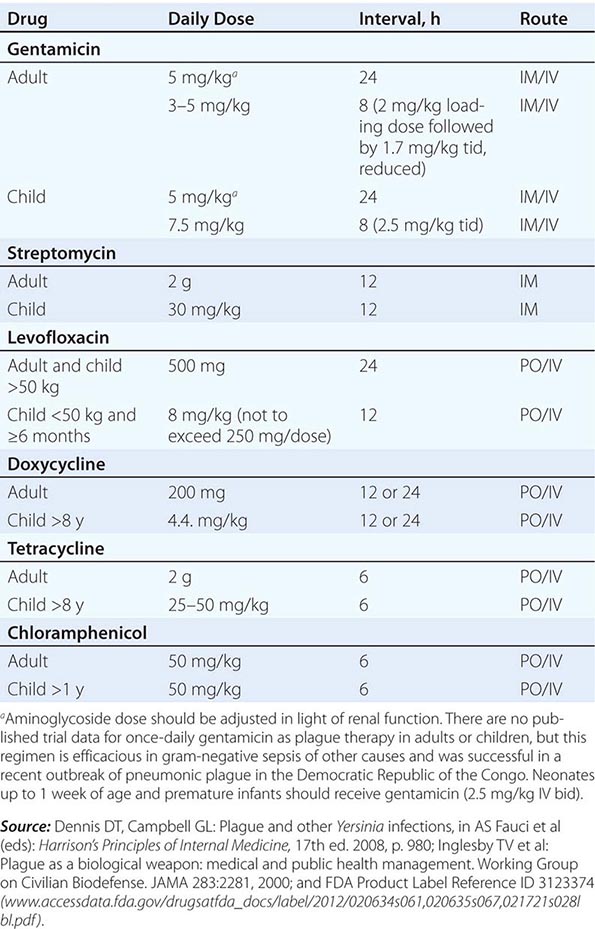
![]() While systemic chloramphenicol therapy is available in the resource-poor countries primarily affected by plague, it is less likely to be available or used in high-income countries because of its adverse effect profile. Tetracyclines are also effective and can be given by mouth but are not recommended for children under the age of 7 years because of tooth discoloration. Doxycycline is the tetracycline of choice; at an oral dosage of 100 mg twice daily, this drug was as effective as IM gentamicin (2.5 mg/kg twice daily) in a trial in Tanzania.
While systemic chloramphenicol therapy is available in the resource-poor countries primarily affected by plague, it is less likely to be available or used in high-income countries because of its adverse effect profile. Tetracyclines are also effective and can be given by mouth but are not recommended for children under the age of 7 years because of tooth discoloration. Doxycycline is the tetracycline of choice; at an oral dosage of 100 mg twice daily, this drug was as effective as IM gentamicin (2.5 mg/kg twice daily) in a trial in Tanzania.
Although Y. pestis is sensitive to β-lactam drugs in vitro and these drugs have been efficacious against plague in some animal models, the response to penicillins has been poor in some clinical cases; thus β-lactams and macrolides are not generally recommended as first-line therapy. Chloramphenicol, alone or in combination, is recommended for some focal complications of plague (e.g., meningitis, endophthalmitis, myocarditis) because of its tissue penetration properties. Fluoroquinolones, effective in vitro and in animal models, are recommended in guidelines for possible bioterrorism-associated pneumonic plague and are increasingly used in therapy, although the only human efficacy data available so far are from a case report. Animal and in vitro studies suggest that fluoroquinolones other than levofloxacin, at doses used in systemic gram-negative sepsis, should be effective as therapy for plague: e.g., ciprofloxacin (400 mg twice daily IV, 500 mg twice daily by mouth), ofloxacin (400 mg twice daily IV or by mouth), or moxifloxacin (400 mg/d IV or by mouth).
PREVENTION
![]() In endemic areas, the control of plague in humans is based on reduction of the likelihood of being bitten by infected fleas or exposed to infected droplets from either humans or animals with plague pneumonia. In the United States, residence and outdoor activity in rural areas of western states where epizootics occur are the main risk factors for infection. To assess potential risks to humans in specific areas, surveillance for Y. pestis infection among animal plague hosts and vectors is carried out regularly as well as in response to observed animal die-offs. Personal protective measures include avoidance of areas where a plague epizootic has been identified and publicized (e.g., by warning signs or closure of campsites). Sick or dead animals should not be handled by the general public. Hunters and zoologists should wear gloves when handling wild-animal carcasses in endemic areas. General measures to avoid rodent fleabite during outdoor activity are appropriate and include the use of insect repellant, insecticide, and protective clothing. General measures to reduce peridomestic and occupational human contact with rodents are advised and include rodent-proofing of buildings and food-waste stores and removal of potential rodent habitats (e.g., woodpiles and junk heaps). Flea control by insecticide treatment of wild rodents is an effective means of minimizing human contact with plague if an epizootic is identified in an area close to human habitation. Any attempt to reduce rodent numbers must be preceded by flea suppression to reduce the migration of infected fleas to human hosts. An oral F1-V subunit vaccine using raccoon poxvirus (RCN) as a vector protects prairie dogs against Y. pestis injections and is being investigated for efficacy in preventing disease in wild animals, thus potentially reducing human exposure.
In endemic areas, the control of plague in humans is based on reduction of the likelihood of being bitten by infected fleas or exposed to infected droplets from either humans or animals with plague pneumonia. In the United States, residence and outdoor activity in rural areas of western states where epizootics occur are the main risk factors for infection. To assess potential risks to humans in specific areas, surveillance for Y. pestis infection among animal plague hosts and vectors is carried out regularly as well as in response to observed animal die-offs. Personal protective measures include avoidance of areas where a plague epizootic has been identified and publicized (e.g., by warning signs or closure of campsites). Sick or dead animals should not be handled by the general public. Hunters and zoologists should wear gloves when handling wild-animal carcasses in endemic areas. General measures to avoid rodent fleabite during outdoor activity are appropriate and include the use of insect repellant, insecticide, and protective clothing. General measures to reduce peridomestic and occupational human contact with rodents are advised and include rodent-proofing of buildings and food-waste stores and removal of potential rodent habitats (e.g., woodpiles and junk heaps). Flea control by insecticide treatment of wild rodents is an effective means of minimizing human contact with plague if an epizootic is identified in an area close to human habitation. Any attempt to reduce rodent numbers must be preceded by flea suppression to reduce the migration of infected fleas to human hosts. An oral F1-V subunit vaccine using raccoon poxvirus (RCN) as a vector protects prairie dogs against Y. pestis injections and is being investigated for efficacy in preventing disease in wild animals, thus potentially reducing human exposure.
Patients in whom pneumonic plague is suspected should be managed in isolation, with droplet precautions observed until pneumonia is excluded or effective antimicrobial therapy has been given for 48 h. Review of the literature published before the advent of antimicrobial agents suggests that the main infective risk is posed by patients in the final stages of disease who are coughing up sputum with plentiful visible blood and/or pus. Cotton and gauze masks were protective in these circumstances. Current surgical masks capable of barrier protection against droplets, including large respiratory particles, are considered protective; a particulate respirator (e.g., N95 or greater) is not required.
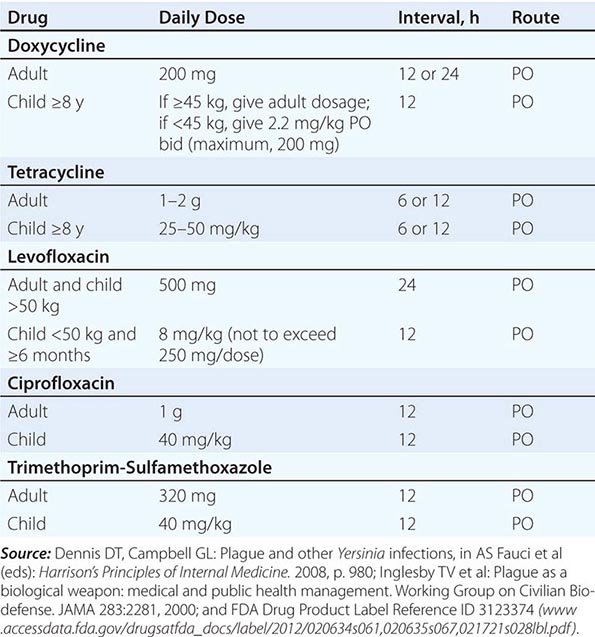
Antimicrobial Prophylaxis Postexposure antimicrobial prophylaxis lasting 7 days is recommended following household, hospital, or other close contact with persons with untreated pneumonic plague. (Close contact is defined as contact with a patient at <2 m.) In animal aerosol-infection studies, levofloxacin and ciprofloxacin are associated with higher survival rates than doxycycline (Table 196-3).
GUIDELINES FOR PLAGUE PROPHYLAXIS |
Immunization Studies with candidate plague vaccines in animal models show that neutralizing antibody provides protection against exposure but that cell-mediated immunity is critical for protection and clearance of Y. pestis from the host. A killed whole-cell vaccine used in humans required multiple doses, caused significant local and systemic reactions, and was not protective against pneumonic plague; this vaccine is not currently available in the United States. A live attenuated vaccine based on strain EV76 is still used in countries of the former Soviet Union but has significant side effects. The vaccines closest to licensure are subunit vaccines comprising recombinant F1 (rF1) and various recombinant V (rV) proteins produced in Escherichia coli, which are combined either as a fusion protein or as a mixture, purified, and adsorbed to aluminum hydroxide for injection. This combination protects mice and various nonhuman primates in laboratory models of bubonic and pneumonic plague and has been evaluated in phase 2 clinical trials. Special ethical considerations with controlled clinical studies involving plague in humans make prelicensure field efficacy studies unlikely. In the United States, the FDA is therefore prepared to assess plague vaccines for human use under the Animal Rule, using efficacy data and other results from animal studies as well as antibodies and other correlates of immunity from human vaccine recipients (www.fda.gov/BiologicsBloodVaccines/ScienceResearch/BiologicsResearchAreas/ucm127288.htm). Live attenuated Y. pseudotuberculosis and Salmonella strains expressing Y. pestis–specific antigens have been shown to be protective in laboratory animal models of bubonic and pneumonic plague and could be delivered by the oral route. A wide variety of other delivery mechanisms for Y. pestis antigens are being explored. Antigens other than F1 and V that could be added to subunit vaccines are being investigated. Advances providing impetus for exploration of these antigens are (1) the recovery of F1-negative Y. pestis strains from natural sources and (2) the observation that F1 antigen is not required for virulence in primate models of pneumonic plague.
YERSINIOSIS
Yersiniosis is a zoonotic infection with an enteropathogenic Yersinia species, usually Yersinia enterocolitica or Y. pseudotuberculosis. The usual hosts for these organisms are pigs and other wild and domestic animals; humans are usually infected by the oral route, and outbreaks from contaminated food occur. Yersiniosis is most common in childhood and in colder climates. Patients present with abdominal pain and sometimes with diarrhea (which may not occur in up to 50% of cases). Y. enterocolitica is more closely associated with terminal ileitis and Y. pseudotuberculosis with mesenteric adenitis, but both organisms may cause mesenteric adenitis and symptoms of abdominal pain and tenderness that result in pseudoappendicitis, with the surgical removal of a normal appendix. Diagnosis is based on culture of the organism or convalescent serology. Y. pseudotuberculosis and some rarer strains of Y. enterocolitica are especially likely to cause systemic infection, which is also particularly common among patients with diabetes or iron overload. Systemic sepsis is treatable with antimicrobial agents, but postinfective arthropathy responds poorly to such therapy. Fourteen other Yersinia species are now recognized, but all lack the virulence plasmid pYV common to Y. pestis, Y. pseudotuberculosis, and Y. enterocolitica and are generally considered to be, at most, opportunistic pathogens of humans (Y. aldovae, Y. aleksiciae, Y. bercovieri, Y. entomophaga, Y. frederiksenii, Y. intermedia, Y. kristensenii, Y. massiliensis, Y. mollaretii, Y. nurmii, Y. pekkanenii, Y. rohdei, Y. similis, and Y. ruckeri). Molecular phylogeny shows that Y. enterocolitica is more distantly related to Y. pseudotuberculosis than these other Yersinia species, and the similar virulence plasmid they share has probably been acquired independently by at least one of the two since the species diverged.
EPIDEMIOLOGY
![]() Y. enterocolitica Y. enterocolitica is found worldwide and has been isolated from a wide variety of wild and domestic animals and environmental samples, including samples of food and water. In vitro, Y. enterocolitica is resistant to predation by the protozoon Acanthamoeba castellanii and can survive inside it, suggesting a possible mode of environmental persistence. Strains are differentiated by combined biochemical reactions (biovar) and serogroup. Most clinical infections are associated with serogroups O:3, O:9, and O:5,27, with a declining number of O:8 infections in North America. Some O:8 infections, previously confined to North America, have been reported from Europe and Japan in recent years, and serogroup O:8 now causes a high percentage of yersiniosis cases in Poland. Yersiniosis, mostly due to Y. enterocolitica, is the third commonest zoonosis reported in Europe; most reports come from northern Europe, especially Germany and Scandinavia. The incidence is highest among children; children under the age of 4 years are more likely to present with diarrhea than are older children. Abdominal pain with mesenteric adenitis and terminal ileitis is more prominent among older children and adults. Septicemia is more likely in patients with preexisting conditions such as diabetes mellitus, liver disease, any condition involving iron overload (including thalassemia and hemochromatosis), advanced age, malignancy, or HIV/AIDS. As in enteritis of other bacterial etiologies, postinfective complications such as reactive arthritis occur mainly in individuals who are HLA-B27 positive. Erythema nodosum (see Fig. 25e-40) following Yersinia infection is not associated with HLA-B27 and is more common among women than among men.
Y. enterocolitica Y. enterocolitica is found worldwide and has been isolated from a wide variety of wild and domestic animals and environmental samples, including samples of food and water. In vitro, Y. enterocolitica is resistant to predation by the protozoon Acanthamoeba castellanii and can survive inside it, suggesting a possible mode of environmental persistence. Strains are differentiated by combined biochemical reactions (biovar) and serogroup. Most clinical infections are associated with serogroups O:3, O:9, and O:5,27, with a declining number of O:8 infections in North America. Some O:8 infections, previously confined to North America, have been reported from Europe and Japan in recent years, and serogroup O:8 now causes a high percentage of yersiniosis cases in Poland. Yersiniosis, mostly due to Y. enterocolitica, is the third commonest zoonosis reported in Europe; most reports come from northern Europe, especially Germany and Scandinavia. The incidence is highest among children; children under the age of 4 years are more likely to present with diarrhea than are older children. Abdominal pain with mesenteric adenitis and terminal ileitis is more prominent among older children and adults. Septicemia is more likely in patients with preexisting conditions such as diabetes mellitus, liver disease, any condition involving iron overload (including thalassemia and hemochromatosis), advanced age, malignancy, or HIV/AIDS. As in enteritis of other bacterial etiologies, postinfective complications such as reactive arthritis occur mainly in individuals who are HLA-B27 positive. Erythema nodosum (see Fig. 25e-40) following Yersinia infection is not associated with HLA-B27 and is more common among women than among men.
Consumption or preparation of raw pork products (such as chitterlings) and some processed pork products is strongly linked with infection because a high percentage of pigs carry pathogenic Y. enterocolitica strains. Outbreaks of Y. enterocolitica infection have been associated with consumption of milk (pasteurized, unpasteurized, and chocolate-flavored) and various foods contaminated with springwater. Person-to-person transmission is suspected in a few cases (e.g., in nosocomial and familial outbreaks) but is much less likely with Y. enterocolitica than with other causes of gastrointestinal infection, such as Salmonella. A multivariate analysis indicates that contact with companion animals is a risk factor for Y. enterocolitica infection among children in Sweden, and low-level colonization of dogs and cats with Y. enterocolitica has been reported. Transfusion-associated septicemia due to Y. enterocolitica, while recognized as a very rare but frequently fatal event for over 30 years, has been difficult to eradicate.
![]() Y. pseudotuberculosis Y. pseudotuberculosis is less frequently reported as a cause of human disease than Y. enterocolitica, and infection with Y. pseudotuberculosis is more likely to present as fever and abdominal pain due to mesenteric lymphadenitis. This organism is associated with wild mammals (rodents, rabbits, and deer), birds, and domestic pigs. Strains are differentiated by combined biochemical reactions (biovar) and serogroup. Although outbreaks are generally rare, several have recently occurred in Finland in association with consumption of lettuce and raw carrots.
Y. pseudotuberculosis Y. pseudotuberculosis is less frequently reported as a cause of human disease than Y. enterocolitica, and infection with Y. pseudotuberculosis is more likely to present as fever and abdominal pain due to mesenteric lymphadenitis. This organism is associated with wild mammals (rodents, rabbits, and deer), birds, and domestic pigs. Strains are differentiated by combined biochemical reactions (biovar) and serogroup. Although outbreaks are generally rare, several have recently occurred in Finland in association with consumption of lettuce and raw carrots.
PATHOGENESIS
The usual route of infection is oral. Studies with both Y. enterocolitica and Y. pseudotuberculosis in animal models suggest that initial replication in the small intestine is followed by invasion of Peyer’s patches of the distal ileum via M cells, with onward spread to mesenteric lymph nodes. The liver and spleen can also be involved after oral infection. The characteristic histologic appearance of enteropathogenic yersiniae after invasion of host tissues is as extracellular microabscesses surrounded by an epithelioid granulomatous lesion.
Experiments involving oral infection of mice with tagged Y. enterocolitica show that only a very small proportion of bacteria in the gut invade tissues. Individual bacterial clones from an orally inoculated pool give rise to each microabscess in a Peyer’s patch, and the host restricts the invasion of previously infected Peyer’s patches. A prior model positing progressive bacterial spread from Peyer’s patches and mesenteric lymph nodes to the liver and spleen appears to be inaccurate: spread of individually tagged clones of Y. pseudotuberculosis to the liver and spleen of mice occurs independently of regional lymph node colonization and in mice lacking Peyer’s patches.
![]() Invasion requires the expression of several nonfimbrial adhesins, such as invasin (Inv) and—in Y. pseudotuberculosis—Yersinia adhesin A (YadA). Inv interacts directly with β1 integrins, which are expressed on the apical surfaces of M cells but not enterocytes. YadA of Y. pseudotuberculosis interacts with extracellular matrix proteins such as collagen and fibronectin to facilitate host cell integrin association and invasion. YadA of Y. enterocolitica lacks a crucial N-terminal region and binds collagen and laminin but not fibronectin and does not cause invasion. Inv is chromosomally encoded, whereas YadA is encoded on the virulence plasmid pYV. YadA helps to confer serum resistance by binding host complement regulators such as factor H and C4-binding protein. Another chromosomal gene, ail (attachment and invasion locus), encodes the extracellular protein Ail, which also confers serum resistance by binding these complement regulators.
Invasion requires the expression of several nonfimbrial adhesins, such as invasin (Inv) and—in Y. pseudotuberculosis—Yersinia adhesin A (YadA). Inv interacts directly with β1 integrins, which are expressed on the apical surfaces of M cells but not enterocytes. YadA of Y. pseudotuberculosis interacts with extracellular matrix proteins such as collagen and fibronectin to facilitate host cell integrin association and invasion. YadA of Y. enterocolitica lacks a crucial N-terminal region and binds collagen and laminin but not fibronectin and does not cause invasion. Inv is chromosomally encoded, whereas YadA is encoded on the virulence plasmid pYV. YadA helps to confer serum resistance by binding host complement regulators such as factor H and C4-binding protein. Another chromosomal gene, ail (attachment and invasion locus), encodes the extracellular protein Ail, which also confers serum resistance by binding these complement regulators.
By binding to host cell surfaces, YadA allows targeting of immune effector cells by the pYV plasmid–encoded type III secretion system (injectisome). As a consequence, the host’s innate immune response is altered; toxins (Yersinia outer proteins, or Yops) are injected into host macrophages, neutrophils, and dendritic cells, affecting signal transduction pathways, resulting in reduced phagocytosis and inhibited production of reactive oxygen species by neutrophils, and triggering apoptosis of macrophages. Other factors functional in invasive disease include yersiniabactin (Ybt), a siderophore produced by some strains of Y. pseudotuberculosis and Y. enterocolitica as well as other Enterobacteriaceae. Yersiniabactin allows bacteria to access iron from saturated lactoferrin during infection and reduces the production of reactive oxygen species by innate immune effector cells, thereby decreasing bacterial killing. Y. pseudotuberculosis and Y. pestis make other siderophores in addition to yersiniabactin.
CLINICAL MANIFESTATIONS
Self-limiting diarrhea is the most common reported presentation in infection with pathogenic Y. enterocolitica, especially in children under the age of 4, who form the single largest group in most case series. Blood may be detected in diarrheal stool. Older children and adults are more likely than younger children to present with abdominal pain, which can be localized to the right iliac fossa—a situation that often leads to laparotomy for presumed appendicitis (pseudoappendicitis). Appendectomy is not indicated for Yersinia infection causing pseudoappendicitis. Thickening of the terminal ileum and cecum is seen on endoscopy and ultrasound, with elevated round or oval lesions that may overlie Peyer’s patches. Mesenteric lymph nodes are enlarged. Ulcerations of the mucosa are noted on endoscopy. Gastrointestinal complications include granulomatous appendicitis, a chronic inflammatory condition affecting the appendix that is responsible for ≤2% of cases of appendicitis; Yersinia is involved in a minority of cases. Y. enterocolitica infection can present as acute pharyngitis with or without other gastrointestinal symptoms. Fatal Y. enterocolitica pharyngitis has been recorded. Mycotic aneurysm can follow Y. enterocolitica bacteremia, as can focal infection (abscess) in many other sites and body compartments (liver, spleen, kidney, bone, meninges, endocardium).
![]() In all age groups, Y. pseudotuberculosis infection is more likely to present as abdominal pain and fever than as diarrhea. A superantigenic toxin—Y. pseudotuberculosis mitogen (YPM)—is produced by strains seen in eastern Russia in association with Far Eastern scarlet-like fever, a childhood illness with desquamating rash, arthralgia, and toxic shock. A similar illness is recognized in Japan (Izumi fever) and Korea. Similarities have been noted with Kawasaki disease, the idiopathic acute systematic vasculitis of childhood. There is an epidemiologic link between exposure of populations to superantigen-positive Y. pseudotuberculosis and an elevated incidence of Kawasaki disease.
In all age groups, Y. pseudotuberculosis infection is more likely to present as abdominal pain and fever than as diarrhea. A superantigenic toxin—Y. pseudotuberculosis mitogen (YPM)—is produced by strains seen in eastern Russia in association with Far Eastern scarlet-like fever, a childhood illness with desquamating rash, arthralgia, and toxic shock. A similar illness is recognized in Japan (Izumi fever) and Korea. Similarities have been noted with Kawasaki disease, the idiopathic acute systematic vasculitis of childhood. There is an epidemiologic link between exposure of populations to superantigen-positive Y. pseudotuberculosis and an elevated incidence of Kawasaki disease.
Y. enterocolitica or Y. pseudotuberculosis septicemia presents as a severe illness with fever and leukocytosis, often without localizing features, and is significantly associated with predisposing conditions such as diabetes mellitus, liver disease, and iron overload. Hemochromatosis combines several of these risk factors. Administration of iron chelators like desferrioxamine, which provide iron accessible to Yersinia (and have an inhibitory effect on neutrophil function), may result in Yersinia septicemia in patients with iron overload who presumably have an otherwise mild gastrointestinal infection. HIV/AIDS has been associated with Y. pseudotuberculosis septicemia. The unusual phenomenon of transfusion-associated septicemia is linked to the ability of Y. enterocolitica to multiply at refrigerator temperature (psychrotrophy). Typically, the transfused unit has been stored for >20 days, and it is believed that small numbers of yersiniae from an apparently healthy donor with subclinical bacteremia are amplified to very high numbers by growth inside the bag at ≤4°C, with consequent septic shock after transfusion. A method for preventing this very rare event (i.e., a range of 1 case in 500,000 to 1 case in several million transfused units in countries such as the United States and France) without unacceptable restriction in the blood supply has not yet been devised.
POSTINFECTIVE PHENOMENA
![]() Like other invasive infections of intestinal origin (salmonellosis, shigellosis), reactive arthritis (articular arthritis of multiple joints developing within 2–4 weeks of a preceding infection) results from autoimmune activity initiated by the deposition of bacterial components (not viable bacteria) in joints in combination with the immune response to invading bacteria. The majority of individuals affected by reactive arthritis due to Yersinia are HLA-B27 positive. Myocarditis with electrocardiographic ST-segment abnormalities may occur with Yersinia-associated reactive arthritis. Most Yersinia-associated cases follow Y. enterocolitica infection (presumably because it is more common than infection with other species), but Y. pseudotuberculosis–associated reactive arthritis is also well documented in Finland, where sporadic and outbreak infections with Y. pseudotuberculosis are more common than in other countries. Of infected individuals identified in a recent Y. pseudotuberculosis serotype O:3 outbreak in Finland, 12% developed reactive arthritis affecting the small joints of the hands and feet, knees, ankles, and shoulders and lasting >6 months in most cases. Erythema nodosum (see Fig. 25e-40) occurs after Yersinia infection (more commonly in women) with no evidence of HLA-B27 linkage.
Like other invasive infections of intestinal origin (salmonellosis, shigellosis), reactive arthritis (articular arthritis of multiple joints developing within 2–4 weeks of a preceding infection) results from autoimmune activity initiated by the deposition of bacterial components (not viable bacteria) in joints in combination with the immune response to invading bacteria. The majority of individuals affected by reactive arthritis due to Yersinia are HLA-B27 positive. Myocarditis with electrocardiographic ST-segment abnormalities may occur with Yersinia-associated reactive arthritis. Most Yersinia-associated cases follow Y. enterocolitica infection (presumably because it is more common than infection with other species), but Y. pseudotuberculosis–associated reactive arthritis is also well documented in Finland, where sporadic and outbreak infections with Y. pseudotuberculosis are more common than in other countries. Of infected individuals identified in a recent Y. pseudotuberculosis serotype O:3 outbreak in Finland, 12% developed reactive arthritis affecting the small joints of the hands and feet, knees, ankles, and shoulders and lasting >6 months in most cases. Erythema nodosum (see Fig. 25e-40) occurs after Yersinia infection (more commonly in women) with no evidence of HLA-B27 linkage.
There is a long-standing association between antithyroid and anti-Yersinia antibodies. Antibody evidence of prior Y. enterocolitica infection in Graves’ disease and increased levels of antithyroid antibody in patients with Y. enterocolitica antibodies were first noted in the 1970s. Y. enterocolitica contains a thyroid-stimulating hormone (TSH)–binding site that is recognized by anti-TSH antibodies from Graves’ disease patients. Raised titers of antibodies to Y. enterocolitica whole cells and Yops have been found in some series of Graves’ disease patients but not in others. One Danish study of twins found no evidence of an association between asymptomatic Yersinia infection (as evidenced by anti-Yop antibody titers) and antithyroid antibodies in euthyroid individuals, while another Danish study of twins with and without Graves’ disease found that increased anti-Yop antibody titers were associated with Graves’ disease. It remains unclear whether this cross-reactivity is significant in the etiology of Graves’ disease.
LABORATORY DIAGNOSIS
Standard laboratory culture methods can be used to isolate enteropathogenic Yersinia species from sterile samples, including blood and cerebrospinal fluid. Culture on specific selective media (CIN agar), with or without pre-enrichment in broth or phosphate-buffered saline at either 4°C or 16°C, is the basis of most schema for isolation of yersiniae from stool or other nonsterile samples. Outside known high-incidence areas, specific culture may be carried out by laboratories only upon request. Virulence plasmid–negative strains of Y. enterocolitica can be isolated from cultures of stool from asymptomatic individuals, especially after cold enrichment. These strains usually differ in biotype (typically biovar 1a) from virulence plasmid–possessing strains; although some display apparent pathogenicity in a mouse model, virulence plasmid–negative strains are not commonly accepted as human pathogens. Because of the frequency with which the virulence plasmid is lost on laboratory subculture, combined biochemical identification (with biotyping according to a standard schema) and serologic identification are usually required to interpret the significance of an isolate of Y. enterocolitica from a nonsterile site. Most pathogenic Y. enterocolitica strains currently isolated from humans are of serogroup O:3/biovar 4 or serogroup O:9/biovar 2; this pattern holds even in the United States, where serogroup O:8/biovar 1B strains were previously predominant. Many self-validated multiplex PCR screens for detection of Y. enterocolitica in clinical samples—and rather more for its detection in food—have been described, but none of these assays is widely used outside its originating laboratory. Some CE-marked real-time PCR kits are now available in Europe for the diagnosis of yersiniosis in animals; as molecular diagnosis of enteric infection becomes more routine in human disease, it is likely that Y. enterocolitica will be included in diagnostic multiplex PCR screens of feces. Because of the presence of Ail in biovar 1a strains, this antigen cannot be used alone in diagnostic assays. A standard for PCR detection in food samples is being prepared by the International Organization for Standardization.
Agglutinating or ELISA antibody titers to specific O-antigen types are used in the retrospective diagnosis of both Y. enterocolitica and Y. pseudotuberculosis infections. IgA and IgG antibodies persist in patients with reactive arthritis. Serologic cross-reactions between Y. enterocolitica serogroup O:9 and Brucella are due to the similarity of their lipopolysaccharide structures. Multiple assays are required to cover even the predominant serogroups (Y. enterocolitica O:3, O:5,27, and O:9; Y. pseudotuberculosis O:1a, O:1b, and O:3), and these assays are generally available only in reference laboratories. ELISA and western blot tests for antibodies to Yops, which are expressed by all pathogenic strains of Y. enterocolitica and Y. pseudotuberculosis, are also available; most of the positivity in these assays probably relates to previous infection with Y. enterocolitica.
TREATMENT | YERSINIOSIS |
Most cases of diarrhea caused by enteropathogenic Yersinia are self-limiting. Data from clinical trials do not support antimicrobial treatment for adults or children with Y. enterocolitica diarrhea. Systemic infections with bacteremia or focal infections outside the gastrointestinal tract generally require antimicrobial therapy. Infants <3 months of age with documented Y. enterocolitica infection may require antimicrobial treatment because of the increased likelihood of bacteremia in this age group. Y. enterocolitica strains nearly always express β-lactamases. Because of the relative rarity of systemic Y. enterocolitica infection, there are no clinical trial data to guide antimicrobial choice or to suggest the optimal dose and duration of therapy. On the basis of retrospective case series and in vitro sensitivity data, fluoroquinolone therapy is effective for bacteremia in adults; for example, ciprofloxacin is given at a typical dose of 500 mg twice daily by mouth or 400 mg twice daily IV for at least 2 weeks (longer if positive blood cultures persist). A third-generation cephalosporin is an alternative—e.g., cefotaxime (typical dose, 6–8 g/d in three or four divided doses). In children, third-generation cephalosporins are effective; for example, cefotaxime is given to children ≥1 month of age at a typical dose of 75–100 mg/kg per day in three or four divided doses, with an increase to 150–200 mg/kg per day in severe cases (maximal daily dose, 8–10 g). Amoxicillin and amoxicillin/clavulanate have shown poor efficacy in case series. Trimethoprim-sulfamethoxazole, gentamicin, and imipenem are all active in vitro. Y. pseudotuberculosis strains do not express β-lactamase but are intrinsically resistant to polymyxin. Because human infection with Y. pseudotuberculosis is less common than that with Y. enterocolitica, less case information is available; however, studies in mice suggest that ampicillin is ineffective. Drugs similar to those used against Y. enterocolitica should be used. The best results have been obtained with a quinolone.
Some trials of treatment for reactive arthritis (with a large proportion of cases due to Yersinia) found that 3 months of oral ciprofloxacin therapy did not affect outcome. One trial in which the same therapy was given specifically for Y. enterocolitica–reactive arthritis found that, while outcome indeed was not affected, there was a trend toward faster remission of symptoms in the treated group. Follow-up 4–7 years after initial antibiotic treatment of reactive arthritis (predominantly following Salmonella and Yersinia infections) demonstrated apparent efficacy in the prevention of chronic arthritis in HLA-B27-positive individuals. A trial showing that azithromycin therapy did not affect outcome in reactive arthritis included cases believed to follow yersiniosis, although no breakdown of cases was provided. A Cochrane review evaluating the use of antibiotics for reactive arthritis is in progress.
PREVENTION AND CONTROL
![]() Current control measures are similar to those used against other enteric pathogens like Salmonella and Campylobacter, which colonize the intestine of food animals. The focus is on safe handling and processing of food. No vaccine is effective in preventing intestinal colonization of food animals by enteropathogenic Yersinia. Consumption of food made from raw pork (which is popular in Germany and Belgium) should be discouraged at present because it is not possible to eliminate contamination with the enteropathogenic Yersinia strains found worldwide in pigs. Exposure of infants to raw pig intestine during domestic preparation of chitterlings is inadvisable. Modification of abattoir technique in Scandinavian countries from the 1990s onward included the removal of pig intestines in a closed plastic bag; levels of carcass contamination with Y. enterocolitica were reduced, but such contamination was not eliminated. Experimental pig herds free of pathogenic Y. enterocolitica O:3 (and also of Salmonella, Toxoplasma, and Trichinella) have been established in Norway and may be commercialized in the future because of their enhanced safety. In the food industry, vigilance is required because of the potential for large outbreaks if small numbers of enteropathogenic yersiniae contaminate any ready-to-eat food whose safe preservation is based on refrigeration before consumption.
Current control measures are similar to those used against other enteric pathogens like Salmonella and Campylobacter, which colonize the intestine of food animals. The focus is on safe handling and processing of food. No vaccine is effective in preventing intestinal colonization of food animals by enteropathogenic Yersinia. Consumption of food made from raw pork (which is popular in Germany and Belgium) should be discouraged at present because it is not possible to eliminate contamination with the enteropathogenic Yersinia strains found worldwide in pigs. Exposure of infants to raw pig intestine during domestic preparation of chitterlings is inadvisable. Modification of abattoir technique in Scandinavian countries from the 1990s onward included the removal of pig intestines in a closed plastic bag; levels of carcass contamination with Y. enterocolitica were reduced, but such contamination was not eliminated. Experimental pig herds free of pathogenic Y. enterocolitica O:3 (and also of Salmonella, Toxoplasma, and Trichinella) have been established in Norway and may be commercialized in the future because of their enhanced safety. In the food industry, vigilance is required because of the potential for large outbreaks if small numbers of enteropathogenic yersiniae contaminate any ready-to-eat food whose safe preservation is based on refrigeration before consumption.
The rare phenomenon of contamination of blood for transfusion has proved impossible to eradicate. However, leukodepletion is now practiced in most blood transfusion centers, primarily to prevent nonhemolytic febrile transfusion reactions and alloimmunization against HLA antigens. This measure reduces but does not eliminate the risk of Yersinia blood contamination.
Notification of yersiniosis is now obligatory in some countries.
197 | Bartonella Infections, Including Cat-Scratch Disease |
Bartonella species are fastidious, facultative intracellular, slow-growing, gram-negative bacteria that cause a broad spectrum of diseases in humans. This genus includes more than 30 distinct species or subspecies, of which at least 16 have been recognized as confirmed or potential human pathogens; Bartonella bacilliformis, Bartonella quintana, and Bartonella henselae are most commonly identified (Table 197-1). Most Bartonella species have successfully adapted to survival in specific domestic or wild mammals. Prolonged intraerythrocytic infection in these animals creates a niche where the bacteria are protected from both innate and adaptive immunity and which serves as a reservoir for human infections. Bartonella characteristically evades the host immune system by modification of its virulence factors (e.g., lipopolysaccharides or flagella) and by attenuation of the immune response. B. bacilliformis and B. quintana, which are not zoonotic, are exceptions. Arthropod vectors are often involved. Isolation and characterization of Bartonella species are difficult and require special techniques. Clinical presentation generally depends on both the infecting Bartonella species and the immune status of the infected individual. Bartonella species are susceptible to many antibiotics in vitro; however, clinical responses to therapy and studies in animal models suggest that the minimal inhibitory concentrations of many antimicrobial agents correlate poorly with the drugs’ in vivo efficacies in patients with Bartonella infections.
BARTONELLA SPECIES KNOWN OR SUSPECTED TO BE HUMAN PATHOGENS |
CAT-SCRATCH DISEASE
DEFINITION AND ETIOLOGY
Usually a self-limited illness, cat-scratch disease (CSD) has two general clinical presentations. Typical CSD, the more common, is characterized by subacute regional lymphadenopathy; atypical CSD is the collective designation for numerous extranodal manifestations involving various organs. B. henselae is the principal etiologic agent of CSD. Rare cases have been associated with Afipia felis and other Bartonella species.
EPIDEMIOLOGY
![]() CSD occurs worldwide, favoring warm and humid climates. In temperate climates, incidence peaks during fall and winter; in the tropics, disease occurs year-round. Adults are affected nearly as frequently as children. Intrafamilial clustering is rare, and person-to-person transmission does not occur. Apparently healthy cats constitute the major reservoir of B. henselae, and cat fleas (Ctenocephalides felis) may be responsible for cat-to-cat transmission. CSD usually follows contact with cats (especially kittens), but other animals (e.g., dogs) have been implicated as possible reservoirs in rare instances. In the United States, the estimated disease incidence is ~10 cases per 100,000 population. About 10% of patients are hospitalized.
CSD occurs worldwide, favoring warm and humid climates. In temperate climates, incidence peaks during fall and winter; in the tropics, disease occurs year-round. Adults are affected nearly as frequently as children. Intrafamilial clustering is rare, and person-to-person transmission does not occur. Apparently healthy cats constitute the major reservoir of B. henselae, and cat fleas (Ctenocephalides felis) may be responsible for cat-to-cat transmission. CSD usually follows contact with cats (especially kittens), but other animals (e.g., dogs) have been implicated as possible reservoirs in rare instances. In the United States, the estimated disease incidence is ~10 cases per 100,000 population. About 10% of patients are hospitalized.
PATHOGENESIS
Inoculation of B. henselae, possibly via contaminated flea feces, usually results from a cat scratch or bite. Infection of mucous membranes or conjunctivae via droplets or licking may occur as well. With lymphatic drainage to one or more regional lymph nodes in immunocompetent hosts, a TH1 response can result in necrotizing granulomatous lymphadenitis. Dendritic cells, along with their associated chemokines, play a role in the host inflammatory response and granuloma formation.
CLINICAL MANIFESTATIONS AND PROGNOSIS
Of patients with CSD, 85–90% have typical disease. The primary lesion, a small (0.3- to 1-cm) painless erythematous papule or pustule, develops at the inoculation site (usually the site of a scratch or a bite) within days to 2 weeks in about one-third to two-thirds of patients (Fig. 197–1A, B). Lymphadenopathy develops 1–3 weeks or longer after cat contact. The affected lymph node(s) are enlarged and usually painful, sometimes have overlying erythema, and suppurate in 10–15% of cases (Fig. 197–1C, D, and E). Axillary/epitrochlear nodes are most commonly involved; next in frequency are head/neck nodes and then inguinal/femoral nodes. Approximately 50% of patients have fever, malaise, and anorexia. A smaller proportion experience weight loss and night sweats mimicking the presentation of lymphoma. Fever is usually low-grade but infrequently rises to ≥39°C. Resolution is slow, requiring weeks (for fever, pain, and accompanying signs and symptoms) to months (for node shrinkage).
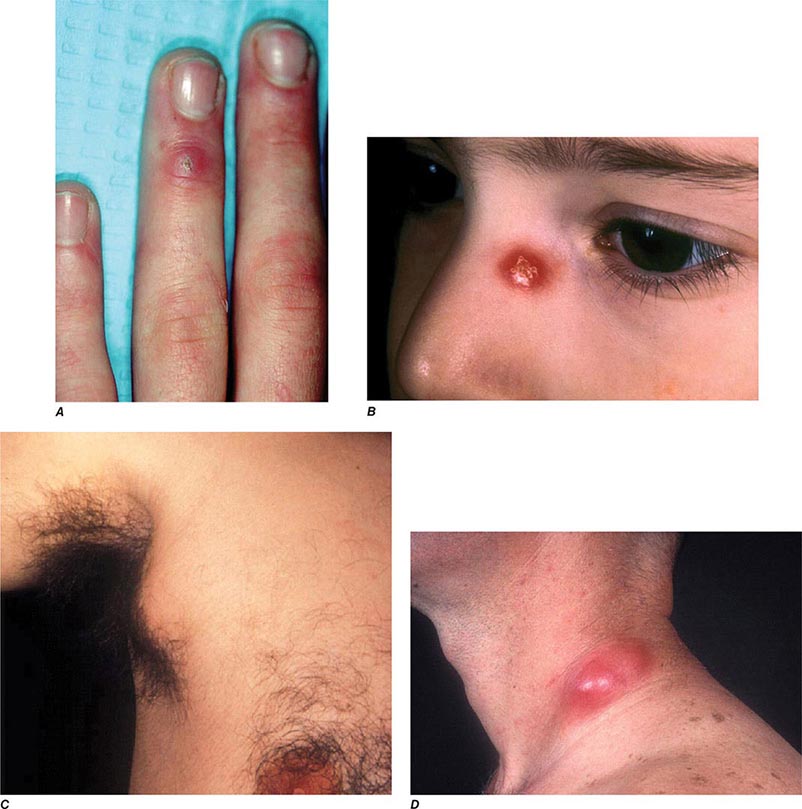
FIGURE 197-1 Manifestations of cat-scratch disease. A. Primary inoculation lesion. Axillary and epitrochlear lymphadenitis appeared 2 weeks later. B. Primary inoculation lesion. Submental lymphadenitis appeared 10 days later. C. Axillary lymphadenopathy of 2 weeks’ duration. The overlying skin appears normal. D. Cervical lymphadenopathy of 6 weeks’ duration. The overlying skin is red. Thick, odorless pus (12 mL) was aspirated. E. Preauricular lymphadenopathy. F. Left-eye neuroretinitis. Note papilledema and stellate macular exudates (“macular star”).
Atypical CSD occurs in 10–15% of patients as extranodal or complicated disease in the absence or presence of lymphadenopathy. Atypical disease includes Parinaud’s oculoglandular syndrome (granulomatous conjunctivitis with ipsilateral preauricular lymphadenitis; Fig. 197–1E), granulomatous hepatitis/splenitis, neuroretinitis (often presenting as unilateral deterioration of vision; Fig. 197–1F), and other ophthalmologic manifestations. In addition, neurologic involvement (encephalopathy, seizures, myelitis, radiculitis, cerebellitis, facial and other cranial or peripheral palsies), fever of unknown origin, debilitating myalgia, arthritis or arthralgia (affecting mostly women >20 years old), osteomyelitis (including multifocal disease), tendinitis, neuralgia, and dermatologic manifestations (including erythema nodosum [see Fig. 25e-40], sometimes accompanying arthropathy) occur. Other manifestations and syndromes (pneumonitis, pleural effusion, idiopathic thrombocytopenic purpura, Henoch-Schönlein purpura, erythema multiforme [see Fig. 25e-25], hypercalcemia, glomerulonephritis, myocarditis) have also been associated with CSD. In elderly patients (>60 years old), lymphadenopathy is more often absent but encephalitis and fever of unknown origin are more common than in younger patients. In immunocompetent individuals, CSD—whether typical or atypical—usually resolves without treatment and without sequelae. Lifelong immunity is the rule.
DIAGNOSIS
Routine laboratory tests usually yield normal or nonspecific results. Histopathology initially shows lymphoid hyperplasia and later demonstrates stellate granulomata with necrosis, coalescing microabscesses, and occasional multinucleated giant cells—findings that, although nonspecific, may narrow the differential diagnosis. Serologic testing (immunofluorescence or enzyme immunoassay) is the most commonly used laboratory diagnostic approach, with variable sensitivity and specificity. Seroconversion may take a few weeks. Other tests are of low sensitivity (culture, Warthin-Starry silver staining), of low specificity (cytology, histopathology), or of limited availability in routine diagnostic laboratories (polymerase chain reaction [PCR], immunohistochemistry). PCR of lymph node tissue, pus, or the primary inoculation lesion is highly sensitive and specific and is particularly useful for definitive and rapid diagnosis in seronegative patients.