Tuberculosis
KEY CONCEPTS
![]() Tuberculosis (TB) is the most prevalent communicable infectious disease on earth. It is the leading cause of death in human immunodeficiency virus (HIV) infection worldwide. It remains out of control in many developing nations. These nations require medical and financial assistance from developed nations in order to control the spread of TB globally.
Tuberculosis (TB) is the most prevalent communicable infectious disease on earth. It is the leading cause of death in human immunodeficiency virus (HIV) infection worldwide. It remains out of control in many developing nations. These nations require medical and financial assistance from developed nations in order to control the spread of TB globally.
![]() In the United States, TB disproportionately affects ethnic minorities as compared with whites, reflecting greater ongoing transmission in ethnic minority communities. Additional TB surveillance and preventive treatment are required within these communities.
In the United States, TB disproportionately affects ethnic minorities as compared with whites, reflecting greater ongoing transmission in ethnic minority communities. Additional TB surveillance and preventive treatment are required within these communities.
![]() Coinfection with HIV and TB accelerates the progression of both diseases, thus requiring rapid diagnosis and treatment of both diseases.
Coinfection with HIV and TB accelerates the progression of both diseases, thus requiring rapid diagnosis and treatment of both diseases.
![]() Mycobacteria are slow-growing organisms; in the laboratory, they require special stains, special growth media, and long periods of incubation to isolate and identify.
Mycobacteria are slow-growing organisms; in the laboratory, they require special stains, special growth media, and long periods of incubation to isolate and identify.
![]() TB can produce atypical signs and symptoms in infants, the elderly, and immunocompromised hosts, and it can progress rapidly in these patients.
TB can produce atypical signs and symptoms in infants, the elderly, and immunocompromised hosts, and it can progress rapidly in these patients.
![]() Latent TB infection (LTBI) can lead to reactivation disease years after the primary infection occurred.
Latent TB infection (LTBI) can lead to reactivation disease years after the primary infection occurred.
![]() The patient suspected of having active TB disease must be isolated until the diagnosis is confirmed and the patient is no longer contagious. Often, isolation takes place in specialized “negative-pressure” hospital rooms to prevent the spread of TB.
The patient suspected of having active TB disease must be isolated until the diagnosis is confirmed and the patient is no longer contagious. Often, isolation takes place in specialized “negative-pressure” hospital rooms to prevent the spread of TB.
![]() Isoniazid and rifampin are the two most important TB drugs; organisms resistant to both these drugs (multidrug-resistant TB [MDR-TB]) are much more difficult to treat.
Isoniazid and rifampin are the two most important TB drugs; organisms resistant to both these drugs (multidrug-resistant TB [MDR-TB]) are much more difficult to treat.
![]() Directly observed treatment (DOT) is considered the standard of care. DOT should be used whenever possible to reduce treatment failures and the selection of drug-resistant isolates.
Directly observed treatment (DOT) is considered the standard of care. DOT should be used whenever possible to reduce treatment failures and the selection of drug-resistant isolates.
![]() Never add a single drug to a failing TB treatment regimen!
Never add a single drug to a failing TB treatment regimen!
INTRODUCTION
![]() Tuberculosis (TB) remains a leading infectious killer globally. TB is caused by Mycobacterium tuberculosis, which can produce either a silent, latent infection or a progressive, active disease.1 Left untreated or improperly treated, TB causes progressive tissue destruction and, eventually, death. Because of renewed public health efforts, TB rates in the United States continue to decline. In contrast, TB remains out of control in many developing countries—to the point that one third of the world’s population currently is infected.1 Given increasing drug resistance, it is critical that a major effort be made to control TB before the most potent drugs are no longer effective.
Tuberculosis (TB) remains a leading infectious killer globally. TB is caused by Mycobacterium tuberculosis, which can produce either a silent, latent infection or a progressive, active disease.1 Left untreated or improperly treated, TB causes progressive tissue destruction and, eventually, death. Because of renewed public health efforts, TB rates in the United States continue to decline. In contrast, TB remains out of control in many developing countries—to the point that one third of the world’s population currently is infected.1 Given increasing drug resistance, it is critical that a major effort be made to control TB before the most potent drugs are no longer effective.
TB rates generally have risen with increasing urbanization and overcrowding because it is easier for an airborne disease to spread when people are packed closely together. Hence, TB became a significant pathogen in Europe during the Middle Ages and peaked during the Industrial Revolution, when it caused approximately 25% of all deaths in Europe and in the United States.1,2 This dire threat led to the rise of public health departments and to procedures such as the isolation of infected patients. Thus, TB was directly responsible for many of the healthcare practices that we take for granted today. Unfortunately, in developing nations, some of these practices are not widely available, and TB continues to rage unabated.
EPIDEMIOLOGY
Globally, roughly 2 billion people are infected by M. tuberculosis, and roughly 2 million people die from active TB each year despite the fact that it is curable.1,2 In the United States, an estimated 9 to 14 million people are latently infected with M. tuberculosis, meaning that they are not currently sick but that they could fall ill with TB at any time. In 2011, a total of 10,521 new TB cases were reported in the United States, an incidence of 3.4 cases per 100,000 population, which is 6.4% lower than the rate in 2010. This is the lowest rate recorded since national reporting began in 1953.3 (For detailed data analysis, visit the Centers for Disease Control and Prevention [CDC] website at www.cdc.gov/nchstp/tb.) The annual incidence of TB in the United States declined by approximately 5% per year from 1953 to 1983 (Fig. 90–1).3 In 1984, this decline slowed, and then the incidence of TB rose from 1988 to 1992, reaching 10.5 cases per 100,000 population. Since 1992, more effective infection control practices and treatment protocols have reduced TB rates significantly as mentioned above. Despite this good news, the eradication of TB from the United States remains very difficult. One reason is that we continue to import new cases from countries where TB remains out of control.3,4
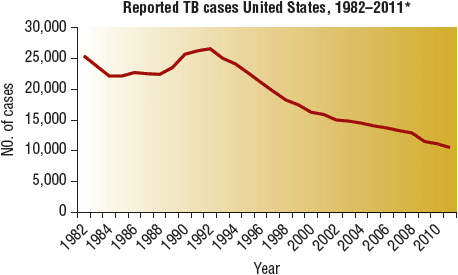
FIGURE 90-1 Reported TB Cases, United States, 1982–2011.
*Updated as of June 25, 2012. (From reference 3.)
Risk Factors for Infection
Location and Place of Birth
Four states (California, Florida, New York, and Texas) continued to report more than 500 cases each in 2011. Combined, these four states accounted for 5,299 TB cases or approximately half (50.4%) of all TB cases reported in 2011.3 Within these states, TB is most prevalent in large urban areas.3
The TB rate among foreign-born persons was 12 times that of U.S.-born persons in 2011.3 The percentage of foreign-born TB patients in the United States has increased annually since 1986, reaching 62.5% in 2011.3 The 17.3 per 100,000 population TB rate among foreign-born persons was a 4.8% decrease since 2010 and a 49% decrease since 1993. In 2011, 54.1% of foreign-born persons with TB originated from five countries: Mexico (1,392 cases), the Philippines (750 cases), Vietnam (537 cases), India (498 cases), and China (365 cases).3 Therefore, healthcare workers must “think TB” when caring for patients from these countries who experience symptoms such as cough, fever, and weight loss.
Close contacts of pulmonary TB patients are most likely to become infected.2–4 These include family members, coworkers, or coresidents in places such as prisons, shelters, or nursing homes. The more prolonged the contact, the greater is the risk, with infection rates as high as 30%.3,4 Although many circumstances exist, TB patients frequently have limited access to healthcare, live in crowded conditions, or are homeless.2–4 Many patients have histories of alcohol abuse or illicit drug use, and many are coinfected with hepatitis B or human immunodeficiency virus (HIV). These concurrent social and health problems make treating some TB patients particularly difficult.
Race, Ethnicity, Age, and Gender
![]() In the United States, TB disproportionately affects ethnic minorities. In 2011, for the first time since the current reporting system began in 1993, non-Hispanic Asians surpassed Hispanics as the largest ethnic group among TB patients. Compared with non-Hispanic whites, the TB rate among non-Hispanic Asians was 25 times greater, and rates among non-Hispanic blacks and Hispanics were 8 and 7 times greater, respectively. Among U.S.-born ethnic groups, the greatest disparity in TB rates occurred among non-Hispanic blacks, whose rate was six times the rate for non-Hispanic whites.3 TB is most common during early adulthood primarily in the 25- to 44-year age group. In 2010, 6% of cases in the United States were children under 15 years of age, 11% were age 15 to 24, 33% were age 25 to 44, 31% were age 45 to 64, and 20% were at least 65 years old.4 TB is more common in older whites and Asians compared with younger people from these groups. This reflects reactivation of latent infection acquired many years earlier when TB was very common. Older blacks and Hispanics also have more TB than younger individuals, but the differences by age are not as pronounced.5 This reflects a greater recent transmission among younger blacks and Hispanics compared with younger whites and Asians. Until the age of 15 years, TB rates are similar for males and females, but after that, the male predominance increases with each decade of life.5
In the United States, TB disproportionately affects ethnic minorities. In 2011, for the first time since the current reporting system began in 1993, non-Hispanic Asians surpassed Hispanics as the largest ethnic group among TB patients. Compared with non-Hispanic whites, the TB rate among non-Hispanic Asians was 25 times greater, and rates among non-Hispanic blacks and Hispanics were 8 and 7 times greater, respectively. Among U.S.-born ethnic groups, the greatest disparity in TB rates occurred among non-Hispanic blacks, whose rate was six times the rate for non-Hispanic whites.3 TB is most common during early adulthood primarily in the 25- to 44-year age group. In 2010, 6% of cases in the United States were children under 15 years of age, 11% were age 15 to 24, 33% were age 25 to 44, 31% were age 45 to 64, and 20% were at least 65 years old.4 TB is more common in older whites and Asians compared with younger people from these groups. This reflects reactivation of latent infection acquired many years earlier when TB was very common. Older blacks and Hispanics also have more TB than younger individuals, but the differences by age are not as pronounced.5 This reflects a greater recent transmission among younger blacks and Hispanics compared with younger whites and Asians. Until the age of 15 years, TB rates are similar for males and females, but after that, the male predominance increases with each decade of life.5
Coinfection with Human Immunodeficiency Virus
![]() HIV is the most important risk factor for active TB, especially among people 25 to 44 years of age.2,4–6 TB and HIV seem to act synergistically within patients and across populations, making each disease worse than it might otherwise be. In 2011, 7.9% of incident cases of TB in the United States were coinfected with HIV.3 This increase in percentage over previous years was felt to be due largely to improved reporting of HIV status to state TB programs. These numbers are estimates because laws and regulations in some states prohibit sharing HIV status of TB patients with the TB program. HIV coinfection may not increase the risk of acquiring M. tuberculosis infection, but it does increase the likelihood of progression to active disease.1,6 There is evidence for higher mortality rates in HIV coinfected with multidrug-resistant (MDR) and extensively drug-resistant (XDR) TB.7
HIV is the most important risk factor for active TB, especially among people 25 to 44 years of age.2,4–6 TB and HIV seem to act synergistically within patients and across populations, making each disease worse than it might otherwise be. In 2011, 7.9% of incident cases of TB in the United States were coinfected with HIV.3 This increase in percentage over previous years was felt to be due largely to improved reporting of HIV status to state TB programs. These numbers are estimates because laws and regulations in some states prohibit sharing HIV status of TB patients with the TB program. HIV coinfection may not increase the risk of acquiring M. tuberculosis infection, but it does increase the likelihood of progression to active disease.1,6 There is evidence for higher mortality rates in HIV coinfected with multidrug-resistant (MDR) and extensively drug-resistant (XDR) TB.7
Risk Factors for Disease
Once infected with M. tuberculosis, a person’s lifetime risk of active TB is approximately 10%.2,4,6 The greatest risk for active disease occurs during the first 2 years after infection. Children younger than 2 years of age and adults older than 65 years of age have two to five times greater risk for active disease compared with other age groups. Patients with underlying immune suppression (e.g., renal failure, cancer, and immunosuppressive drug treatment) have 4 to 16 times greater risk than other patients. Finally, HIV-infected patients with M. tuberculosis infection are 100 times more likely to develop active TB than normal hosts.4,8 HIV-infected patients have an annual risk of active TB of approximately 10%, rather than a lifetime risk at that rate. Therefore, all patients with HIV infection should be screened for tuberculous infection, and those known to be infected with M. tuberculosis should be tested for HIV infection.
ETIOLOGY
M. tuberculosis is a slender bacillus with a waxy outer layer.2,6 It is 1 to 4 μm in length, and under the microscope, it is either straight or slightly curved in shape.1,9,10 It does not stain well with Gram stain, so the Ziehl-Neelsen stain or the fluorochrome stain must be used instead.1,2,6 After Ziehl-Neelsen staining with carbol-fuchsin, mycobacteria retain the red color despite acid–alcohol washes. Hence, they are called acid-fast bacilli (AFB).9 After staining, microscopic examination (“smear”) detects about 8,000 to 10,000 organisms per milliliter (8 × 106/L to 10 × 106/L) of specimen, so a patient can be “smear negative” but still grow M. tuberculosis on culture. Microscopic examination also cannot determine which of the more than 100 mycobacterial species is present or whether the organisms in the original samples were alive or dead.1,9,10 On smear, they are all dead. On culture, M. tuberculosis grows slowly, doubling about every 20 hours. This is slow compared with gram-positive and gram-negative bacteria, which double about every 30 minutes.
Culture and Susceptibility Testing
![]() Direct susceptibility testing involves inoculating specialized media with organisms taken directly from a concentrated, smear-positive specimen.1,9,10 This approach produces susceptibility results in 2 to 3 weeks. Indirect susceptibility testing involves inoculating the test media with organisms obtained from a pure culture of the organisms, which can take several more weeks. The most common agar method, known as the proportion method, uses the ratio of colony counts on drug-containing agar to that on drug-free agar.1,10 In the United States, the critical proportion for resistance is 1%. That means that if a drug-containing plate shows only 2% of the growth seen on a drug-free plate, some of the organisms from the specimen were resistant to that drug. Therefore, it is likely that many of the organisms in the patient also are resistant to that drug, and it should not be used to treat that patient.
Direct susceptibility testing involves inoculating specialized media with organisms taken directly from a concentrated, smear-positive specimen.1,9,10 This approach produces susceptibility results in 2 to 3 weeks. Indirect susceptibility testing involves inoculating the test media with organisms obtained from a pure culture of the organisms, which can take several more weeks. The most common agar method, known as the proportion method, uses the ratio of colony counts on drug-containing agar to that on drug-free agar.1,10 In the United States, the critical proportion for resistance is 1%. That means that if a drug-containing plate shows only 2% of the growth seen on a drug-free plate, some of the organisms from the specimen were resistant to that drug. Therefore, it is likely that many of the organisms in the patient also are resistant to that drug, and it should not be used to treat that patient.
The proportion method’s limitations include many weeks to obtain results, drug degradation during the incubation, and a qualitative result (susceptible or resistant). The newer mycobacterial growth indicator tube (MGIT) (Becton Dickson, Sparks, MD) systems use liquid media and detect live mycobacteria in as few as 9 to 14 days.11,12
Rapid-identification tests are now available. Nucleic acid probes such as the AccuProbe (Gen-Probe, San Diego, CA) use DNA probes to identify the presence of complementary ribosomal ribonucleic acid (rRNA) for several mycobacterial species.6,9,13 DNA fingerprinting using restriction-fragment-length polymorphism analysis has been used to identify clusters of cases.1,9,13 Amplification of the genetic material can be achieved through polymerase chain reaction (PCR) (Roche Molecular Systems, Branchburg, NJ), the amplified M. tuberculosis direct (MTD) test (Gen-Probe), and strand-displacement amplification (SDA; Becton-Dickinson, Sparks, MD).9,12 Thin-layer chromatography, high-performance liquid chromatography for mycolic acid identification, and gas chromatography for short-chain fatty acids (methyl esters) have been used to speciate mycobacterial isolates.1,9,13 Other tests are designed to detect common genetic changes associated with drug resistance, such as changes in the katG gene associated with isoniazid resistance and the rpoB gene associated with rifampin resistance.6,12,14,15 Two tests, the Gene X-pert (Cepheid, Sunnyvale, CA) and the Hain test (Hain Lifescience, Nehren, Germany), have entered into limited clinical use in the United States. These tests offer clinicians a chance to know rapidly if resistance to rifampin is present (both tests) and what drugs might be good initial choices (Hain Test).
Transmission
M. tuberculosis is transmitted from person to person by coughing or other activities that cause the organism to be aerosolized.2,6,11 These particles, called droplet nuclei, contain one to three bacilli and are small enough (1 to 5 mm) to reach the alveolar surface. This produces “droplet nuclei” that are dispersed in the air. Each droplet nuclei contains one to three organisms. Approximately 30% of individuals who experience prolonged contact with an infectious TB patient will become infected.
A person with cavitary, pulmonary TB and a cough may infect roughly one person per month until that person is treated effectively, although this number can vary significantly. A person with the uncommon laryngeal form of TB can spread organisms even when talking, so the transmission rates can be even higher.
PATHOPHYSIOLOGY
Immune Response
T-lymphocyte responses are essential to controlling M. tuberculosis infections.2,6,16,17 In the mouse model, two different T-cell responses—the T-helper type 1 (TH1) response and the T-helper type 2 (TH2) response—have been described. The TH1 response is the preferred response to TB, and the TH2 response, including the potentially subversive influence of interleukin (IL) 4, is undesirable.2,16,17 Some workers have argued that this dichotomy is clearer in the mouse model, and in many humans, the T-cell response may be classified as TH0 (elements of both TH1 and TH2).16 In either case, T lymphocytes activate macrophages that, in turn, engulf and kill mycobacteria. T lymphocytes also destroy immature macrophages that harbor M. tuberculosis but are unable to kill the invaders.16,17 CD4+ cells are the primary T cells involved, with contributions by γδ T cells and CD8+ T cells.20 CD4+ T cells produce interferon-γ (INF-γ) and other cytokines, including IL-2 and IL-10, that coordinate the immune response to TB.16 Because CD4+ cells are depleted in HIV-infected patients, these patients are unable to mount an adequate defense to TB.16,17
Although B-cell responses and antibody production can be demonstrated in TB-infected mammals, these humoral responses do not appear to contribute much to the control of TB within the host.2,6,16 Tumor necrosis factor-α (TNF-α) and INF-γ are important cytokines involved in coordinating the host’s cell-mediated response. Rheumatoid arthritis patients treated with TNF-α inhibitors (such as infliximab) have high rates of reactivation TB.18 Therefore, patients known to be deficient in the activity of TNF-α or INF-γ should be screened for TB infection and offered appropriate treatment.
M. tuberculosis has several ways of evading or resisting the host immune response.16,17 In particular, M. tuberculosis can inhibit the fusion of lysosomes to phagosomes inside macrophages. This prevents the destructive enzymes found in the lysosomes from getting to the bacilli captured in the phagosomes. This inhibition of destructive mechanisms allows time for M. tuberculosis to escape into the cytoplasm. Virulent M. tuberculosis is able to multiply in the macrophage cytoplasm, thus perpetuating their spread. Finally, lipoarabinomannan (LAM), the principal structural polysaccharide of the mycobacterial cell wall, inhibits the host immune response.16,17 LAM induces immunosuppressive cytokines, thus blocking macrophage activation; additionally, LAM scavenges O2, thus preventing attack by superoxide anions, hydrogen peroxide, singlet oxygen, and hydroxyl radicals.16,17 These survival mechanisms make M. tuberculosis a particularly difficult organism to control. Any defects in the host immune system make it likely that M. tuberculosis will not be controlled and that active disease will ensue.
Primary Infection
Primary infection usually results from inhaling airborne particles that contain M. tuberculosis.2,6,17 The progression to clinical disease depends on three factors: (a) the number of M. tuberculosis organisms inhaled (infecting dose), (b) the virulence of these organisms, and (c) the host’s cell-mediated immune response.2,3,6,11,17,19 At the alveolar surface, the bacilli that were delivered by the droplet nuclei are ingested by pulmonary macrophages.22 If these macrophages inhibit or kill the bacilli, infection is aborted.17 If the macrophages cannot do this, the organisms continue to multiply. The macrophages eventually rupture, releasing many bacilli, and these mycobacteria are then phagocytized by other macrophages. This cycle continues over several weeks until the host is able to mount a more coordinated response.17 During this early phase of infection, M. tuberculosis multiplies logarithmically.17
Some of the intracellular organisms are transported by the macrophages to regional lymph nodes in the hilar, mediastinal, and retroperitoneal areas. The cycle of phagocytosis and cell rupture continues. During lymph node involvement, the mycobacteria may be held in check. More frequently, M. tuberculosis spreads throughout the body through the bloodstream.2,6,17 When this intravascular dissemination occurs, M. tuberculosis can infect any tissue or organ in the body. Most commonly, M. tuberculosis infects the posterior apical region of the lungs. This may be so because of the high oxygen content, and it may be because of a less vigorous immune response in this area.
After about 3 weeks of infection, T lymphocytes are presented with M. tuberculosis antigens. These T cells become activated and begin to secrete INF-γ and the other cytokines noted earlier. The processes described in Immune Response above then begin to occur. First, T lymphocytes stimulate macrophages to become bactericidal.17 Large numbers of activated microbicidal macrophages surround the solid caseous (cheese-like) tuberculous foci (the necrotic area of infection).17 This process of creating activated microbicidal macrophages is known as cell-mediated immunity (CMI).17
At the same time that CMI occurs, delayed-type hypersensitivity (DTH) also develops through the activation and multiplication of T lymphocytes. DTH refers to the cytotoxic immune process that kills nonactivated immature macrophages that are permitting intracellular bacillary replication.17 These immature macrophages are killed when the T lymphocytes initiate Fas-mediated apoptosis (programmed cell death).17 The bacilli released from the immature macrophages then are killed by the activated macrophages.17
By this time (>3 weeks), macrophages have begun to form granulomas to contain the organisms. In a typical tuberculous granuloma, activated macrophages accumulate around a caseous lesion and prevent its further extension.17 At this point, the infection is largely under control, and bacillary replication falls off dramatically. Depending on the inflammatory response, tissue necrosis and calcification of the infection site plus the regional lymph nodes may occur.
Over 1 to 3 months, activated lymphocytes reach an adequate number, and tissue hypersensitivity results. This is shown by a positive tuberculin skin test. Any remaining mycobacteria are believed to reside primarily within granulomas or within macrophages that have avoided detection and lysis, although some residual bacilli have been found in various types of cells.2,6,16
Approximately 90% of infected patients have no further clinical manifestations. Most patients only show a positive skin test (70%), whereas some also have radiographic evidence of stable granulomas. This radiodense area on chest radiograph is called a Ghon’s complex. Approximately 5% of patients (usually children, the elderly, and the immunocompromised) experience “progressive primary” disease that occurs before skin test conversion, which presents as a progressive pneumonia, usually in the lower lobes.20 Disease frequently spreads, leading to meningitis and other severe forms of TB.20 Because of this risk of severe disease, very young, elderly, and immunocompromised patients, including those with HIV, should be evaluated and treated for latent or active TB.
Reactivation Disease
![]() Roughly 10% of infected patients develop reactivation disease at some point in their lives. Nearly half of these cases occur within 2 years of infection.2,6,11 In the United States, most cases of TB are believed to result from reactivation. Reinfection is uncommon in the United States because of the low rate of exposure and because previously sensitized individuals possess some degree of immunity to reinfection.2,17 Exceptions include patients coinfected with HIV who live in areas of higher exposure to M. tuberculosis.
Roughly 10% of infected patients develop reactivation disease at some point in their lives. Nearly half of these cases occur within 2 years of infection.2,6,11 In the United States, most cases of TB are believed to result from reactivation. Reinfection is uncommon in the United States because of the low rate of exposure and because previously sensitized individuals possess some degree of immunity to reinfection.2,17 Exceptions include patients coinfected with HIV who live in areas of higher exposure to M. tuberculosis.
The apices of the lungs are the most common sites for reactivation (85% of cases).2 For reasons that are not entirely known (waning cellular immunity, loss of specific T-cell clones, blocking antibody), organisms within granulomas emerge and begin multiplying extracellularly.17 The inflammatory response produces caseating granulomas, which eventually will liquefy and spread locally, leading to the formation of a hole (cavity) in the lungs.
The immune response contributes to the severity of the lung damage, and DTH allows for intracellular mycobacterial multiplication.16,17 In addition, there is “innocent bystander” killing of host cells and locally thrombosed blood vessels.17 The killing of mycobacteria, macrophages, and neutrophils that have entered the battle releases cytokines and lysozymes into the infectious foci. This toxic mixture can be too much for the surrounding alveoli and airway cells, causing regional necrosis and structural collapse.2,17 These unstable foci liquefy, spreading the infection to neighboring areas of the lung, creating a cavity. Some of this necrotic material is coughed out, producing droplet nuclei. Bacterial counts in the cavities can be as high as 108 per milliliter of cavitary fluid. Partial healing may result from fibrosis, but these lesions remain unstable and may continue to expand.2,17 If left untreated, pulmonary TB continues to destroy the lungs, resulting in hypoxia, respiratory acidosis, and eventually death.
Extrapulmonary and Miliary Tuberculosis
Caseating granulomas at extrapulmonary sites can undergo liquefaction, releasing tubercle bacilli and causing symptomatic disease.2,6 Extrapulmonary TB without concurrent pulmonary disease is uncommon in normal hosts but more common in HIV-infected patients. Because of these unusual presentations, the diagnosis of TB is difficult and often delayed in immunocompromised hosts.2,6 Lymphatic and pleural diseases are the most common forms of extrapulmonary TB, followed by bone, joint, genitourinary, meningeal, and other forms.2,6 Occasionally, a massive inoculum of organisms enters the bloodstream, causing a widely disseminated form of the disease known as miliary TB. It is named for the millet seed appearance of the small granulomas seen on chest radiographs, and it can be rapidly fatal.16 Miliary TB is a medical emergency requiring immediate treatment.
Influence of HIV Infection on Pathogenesis
![]() HIV infection is the strongest single risk factor for active TB.2,6,16 As CD4+ lymphocytes multiply in response to the mycobacterial infection, HIV multiplies within these cells and selectively destroys them. In turn, the TB-fighting lymphocytes are depleted.16 This vicious cycle puts HIV-infected patients at 100 times the risk of active TB compared with HIV-negative people.21 In addition, the combination of HIV infection and certain social behaviors increases the risk of newly acquired TB. In select areas of the United States during the resurgence of TB during the early 1990s, up to 50% of new TB cases were the result of recent infection, particularly among HIV-infected individuals.21,22
HIV infection is the strongest single risk factor for active TB.2,6,16 As CD4+ lymphocytes multiply in response to the mycobacterial infection, HIV multiplies within these cells and selectively destroys them. In turn, the TB-fighting lymphocytes are depleted.16 This vicious cycle puts HIV-infected patients at 100 times the risk of active TB compared with HIV-negative people.21 In addition, the combination of HIV infection and certain social behaviors increases the risk of newly acquired TB. In select areas of the United States during the resurgence of TB during the early 1990s, up to 50% of new TB cases were the result of recent infection, particularly among HIV-infected individuals.21,22
As mycobacteria spread throughout the body, HIV replication accelerates in lymphocytes and macrophages. This leads to progression of HIV disease.16,23 HIV-infected patients who are infected with TB deteriorate more rapidly unless they receive antimycobacterial chemotherapy.24,25 Most clinicians now recommend beginning TB treatment first, and shortly after, beginning HIV treatment. However, this needs to be individualized based on degree of immunosuppression from HIV and the patient’s tolerance of the treatment regimen. Some patients will experience paradoxical worsening of the TB.11,23 This appears to result from a reinvigorated inflammatory response to TB. Because TB can be very dangerous in HIV-positive patients, they should be screened for tuberculous infection or disease soon after they are shown to be HIV-positive.2,6,16
CLINICAL PRESENTATION
The classical presentation of TB is shown in Clinical Presentation of Tuberculosis above. The onset of TB may be gradual, and the diagnosis may not be considered until a chest radiograph is performed. Unfortunately, many patients do not seek medical attention until more dramatic symptoms, such as hemoptysis, occur. At this point, patients typically have large cavitary lesions in the lungs. These cavities are loaded with M. tuberculosis. Expectoration or swallowing of infected sputum may spread the disease to other areas of the body.1,2,6,19 Physical examination is nonspecific but suggestive of progressive pulmonary disease. ![]() Patients coinfected with HIV may have atypical presentations.1,2,6,19 As their CD4+ counts decline, HIV-positive patients are less likely to have positive skin tests, cavitary lesions, or fever. Pulmonary radiographic findings may be minimal or absent. HIV-positive patients have a higher incidence of extrapulmonary TB and are more likely to present with progressive primary disease. Because their symptoms are not specific to TB, a thorough workup for TB is essential.2,6,16,19
Patients coinfected with HIV may have atypical presentations.1,2,6,19 As their CD4+ counts decline, HIV-positive patients are less likely to have positive skin tests, cavitary lesions, or fever. Pulmonary radiographic findings may be minimal or absent. HIV-positive patients have a higher incidence of extrapulmonary TB and are more likely to present with progressive primary disease. Because their symptoms are not specific to TB, a thorough workup for TB is essential.2,6,16,19
CLINICAL PRESENTATION Tuberculosis
Extrapulmonary TB typically presents as a slowly progressive decline in organ function.2,6,19 Patients may have low-grade fever and other constitutional symptoms. Patients with genitourinary TB may present with sterile pyuria and hematuria. Lymphadenitis often involves the cervical and supraclavicular nodes and may appear as a neck mass with spontaneous drainage. Tuberculous arthritis and osteomyelitis occur most commonly in the elderly and usually affect the lower spine and weight-bearing joints. TB of the spine is known as Pott’s disease.2 Abnormal behavior, headaches, or convulsions suggest tuberculous meningitis. Involvement of the peritoneum, pericardium, larynx, and adrenal glands also occurs.2,6,19
The Elderly
![]() TB in the elderly is easily confused with other respiratory diseases. Many clinical findings are muted or absent altogether. Compared with younger patients, TB in the elderly is far less likely to present with positive skin tests, fevers, night sweats, sputum production, or hemoptysis.2,19,24 Weight loss may occur but is nonspecific. In contrast, mental status changes are twice as common in the elderly, and mortality is six times higher.2,19,24 TB is a preventable cause of death in the elderly that should not be overlooked.
TB in the elderly is easily confused with other respiratory diseases. Many clinical findings are muted or absent altogether. Compared with younger patients, TB in the elderly is far less likely to present with positive skin tests, fevers, night sweats, sputum production, or hemoptysis.2,19,24 Weight loss may occur but is nonspecific. In contrast, mental status changes are twice as common in the elderly, and mortality is six times higher.2,19,24 TB is a preventable cause of death in the elderly that should not be overlooked.
Children
![]() TB in children, especially those younger than 12 years of age, may present as a typical bacterial pneumonia and is called progressive primary TB.19,20 Clinical disease often begins 1 to 2 months after exposure and precedes skin-test positivity. Unlike adults, pulmonary TB in children often involves the lower and middle lobes.19,20 Dissemination to the lymph nodes, GI and genitourinary tracts, bone marrow, and meninges is common. Because of delays in recruitment of cellular immunity, cavitary disease is infrequent, and the number of organisms present typically is smaller than in an adult. Because cavitary lesions are uncommon, children do not spread TB readily. However, TB can be rapidly fatal in a child, and it requires prompt chemotherapy.
TB in children, especially those younger than 12 years of age, may present as a typical bacterial pneumonia and is called progressive primary TB.19,20 Clinical disease often begins 1 to 2 months after exposure and precedes skin-test positivity. Unlike adults, pulmonary TB in children often involves the lower and middle lobes.19,20 Dissemination to the lymph nodes, GI and genitourinary tracts, bone marrow, and meninges is common. Because of delays in recruitment of cellular immunity, cavitary disease is infrequent, and the number of organisms present typically is smaller than in an adult. Because cavitary lesions are uncommon, children do not spread TB readily. However, TB can be rapidly fatal in a child, and it requires prompt chemotherapy.
DIAGNOSIS
Diagnostic Testing
The key to stopping the spread of TB is early identification of infected individuals.1,2,6,19 Table 90–1 lists the populations most likely to benefit from testing (column 1 patients are at highest risk for TB, followed by those in column 2). Members of these high-risk groups should be tested for TB infection and educated about the disease.
TABLE 90-1 Criteria for Tuberculin Positivity by Risk Group
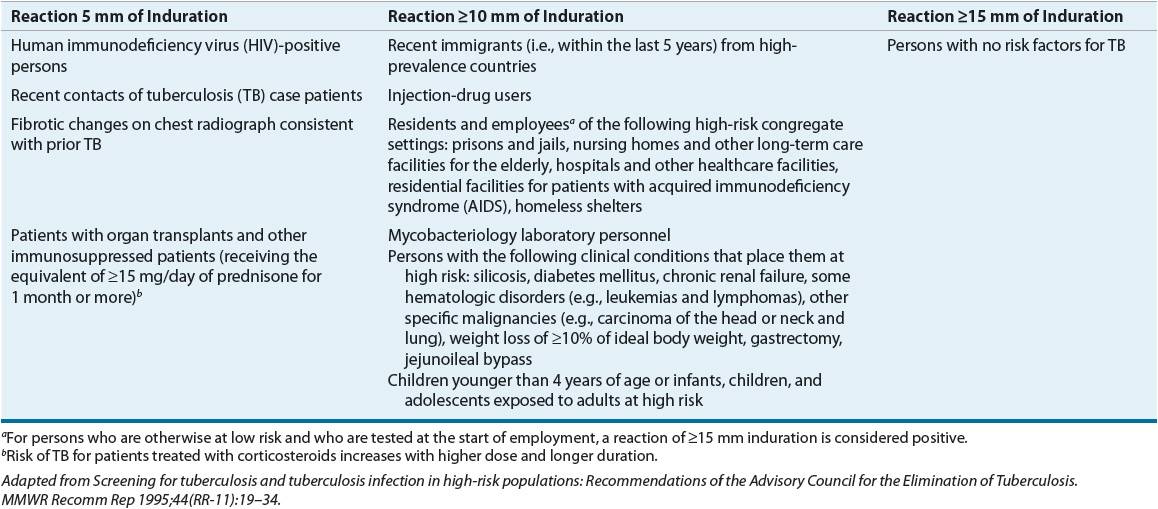
The Mantoux test is a TB skin test. It uses tuberculin purified protein derivative (PPD), and unlike the Heaf or tine test, the Mantoux test is quantitative. The standard 5-tuberculin-unit PPD dose is placed intracutaneously on the volar aspect of the forearm with a 26- or 27-gauge needle.2,19,24 This injection should produce a small, raised, blanched wheal. An experienced professional should read the test in 48 to 72 hours. The area of induration (the “bump”) is the important end point, not the area of redness. Table 90–1 lists the criteria for interpretation.1,2,6,19,24 The CDC does not recommend the routine use of anergy panels.24,26 Aplisol and Tubersol 5-tuberculin-unit products are available commercially and are similar in sensitivity, specificity, and reactivity. It is important, however, to use one product and notify appropriate users when switching between products.27,28
The “booster effect” occurs for patients who do not respond to an initial skin test but show a positive reaction if retested about a week later.19,26 Patients with past M. tuberculosis infection and some patients with past immunization with bacillus Calmette-Guérin (BCG) vaccine or past infection with other mycobacteria may “boost” with a second skin test. Individuals who require periodic skin testing, such as healthcare workers, should receive a two-stage test initially.19,26,29 Once they are shown to be skin-test negative, any positive skin test later shows recent infection, and this requires treatment.
The PPD skin test is an imperfect diagnostic tool. Up to 20% of patients with active TB are falsely skin-test negative, presumably because their immune systems are overwhelmed.16,26 False-positive results are more common in low-risk patients and those recently vaccinated with BCG. Despite BCG vaccination, one should not ignore a positive PPD result. These patients require careful evaluation for active disease, and they may be offered preventive treatment because many come from areas where TB infection is common.
Interferon-γ release assays (IGRA) measure the release of INF-γ in blood in response to the TB antigens.30 They may provide quick and specific results for identifying M. tuberculosis. IGRAs do not trigger a booster effect and are more specific for testing M. tuberculosis than the PPD. The QuantiFERON-TB Gold test (QFT-G) is an enzyme-linked immunosorbent assay (ELISA) and was approved by the U.S. FDA in 2005.30 The T-SPOT.TB, an enzyme-linked immunospot assay, was approved by the U.S. FDA in 2008.31 Both tests can be used for diagnosing latent TB infection (LTBI) and TB disease caused by M. tuberculosis. The antigenic proteins are absent from BCG vaccine strains and from most non-TB mycobacteria. Therefore, QFT-G does not trigger a booster effect and is more specific for testing of M. tuberculosis than the PPD. Although these tests can provide results to diagnose both latent infection and disease, they cannot differentiate between the two. Results are available within <24 hours, instead of the 2 to 3 days required for the traditional PPD skin test. Therefore, the patient does not have to return to the clinic as required by the PPD skin test, making it more convenient. The CDC has approved the use of these tests in all circumstances in which the PPD is currently used; however, the sensitivity for young children (<5 years) and in immunocompromised patients has not been determined.32–35
Additional Tests
When active TB is suspected, attempts should be made to isolate M. tuberculosis from the site of infection.2,6,19,26 Sputum collected in the morning usually has the highest yield.2,9,19 Daily sputum collection over 3 consecutive days is recommended.
For patients unable to expectorate, sputum induction with aerosolized hypertonic saline may produce a diagnostic sample. Bronchoscopy, or aspiration of gastric fluid via a nasogastric tube, may be attempted for select patients.19 For patients with suspected extrapulmonary TB, samples of draining fluid, biopsies of the infected site, or both may be attempted. Blood cultures are positive occasionally, especially in AIDS patients.19,36
TREATMENT
Desired Outcomes
The desired outcomes during the treatment of TB are:
1. Rapid identification of a new TB case
2. Initiation of specific anti-TB treatment
3. Prompt resolution of the signs and symptoms of disease
4. Achievement of a noninfectious state in the patient, thus ending isolation
5. Adherence to the treatment regimen by the patient
6. Cure of the patient as quickly as possible (generally at least 6 months of treatment)
It is also important that patients with active disease are isolated to prevent spread of the disease and that appropriate samples for smears and cultures are collected. Secondary goals are identification of the index case that infected the patient, identification of all persons infected by both the index case and the new case of TB (“contact investigation”), and completion of appropriate treatments for those individuals.
General Approaches to Treatment
Drug treatment is the cornerstone of TB management.2,6,11,37 Monotherapy can be used only for infected patients who do not have active TB (latent infection, as shown by a positive skin test). Once active disease is present, a minimum of two drugs, and generally three or four drugs, must be used simultaneously.2,6,11,37 The duration of treatment depends on the condition of the host, extent of disease, presence of drug resistance, and tolerance of medications. The shortest duration of treatment generally is 6 months, and 2 to 3 years of treatment may be necessary for cases of MDR-TB.2,6,11,37 Because the duration of treatment is so long and because many patients feel better after a few weeks of treatment, careful followup is required. Directly observed therapy (DOT) by a healthcare worker is a cost-effective way to ensure completion of treatment and is considered the standard of care.2,6,11,37–39
Principles for Treating Latent Infection and for Treating Disease
Asymptomatic patients with tuberculous infection have a bacillary load of about 103 organisms, compared with 1011 organisms in a patient with cavitary pulmonary TB.2,6 As the number of organisms increases, the likelihood of naturally occurring drug-resistant mutants also increases. Naturally occurring resistant mutants are found at rates of 1 in 106 to 1 in 108 organisms for the anti-TB drugs.2,37 When treating asymptomatic latent infection with isoniazid monotherapy, the risk of selecting out isoniazid-resistant organisms is low. The isoniazid mutation rate is about 1 in 106, but only about 103 organisms are present in the body. In contrast, the risk of selecting out isoniazid-resistant organisms is unacceptably high for patients with cavitary TB. One can prevent selection of these resistant mutants by adding more drugs because the rates for resistance mutations to multiple drugs are additive functions of the individual rates. For example, only 1 in 1013 organisms would be naturally resistant to both isoniazid (1 in 106) and rifampin (1 in 107).2,37 It is unlikely that such rare organisms are present in a previously untreated patient.
Combination chemotherapy is required for treating active TB disease. The patient should receive at least two drugs to which the isolate is susceptible, and, generally, four drugs are given at the outset of treatment. Rifampin and isoniazid are the best drugs for preventing drug resistance, followed by ethambutol, streptomycin, and pyrazinamide.2,6,37,40
Three subpopulations of mycobacteria are proposed to exist within the body, and each appears to respond to certain drugs.2,37 Most numerous are the extracellular, rapidly dividing bacteria, often found within cavities (about 107 to 109 organisms). These are killed most readily by isoniazid, followed by rifampin, streptomycin, and the other drugs. A second group resides within caseating granulomas (possibly 105 to 107 organisms). These organisms appear to be in a semidormant state, with occasional bursts of metabolic activity. Pyrazinamide, through its conversion within M. tuberculosis to pyrazinoic acid, appears most active against these organisms. Rifampin and isoniazid also may be active against this subpopulation. The third subset is the intracellular mycobacteria present within macrophages (104 to 106). Rifampin, isoniazid, and the quinolones appear to be most active against intracellular M. tuberculosis. While this appears to explain what happens during the treatment of TB, there is no practical way to quantitate these populations within a given patient.
Nonpharmacologic Therapy
![]() Nonpharmacologic interventions aim to (a) prevent the spread of TB, (b) find where TB has already spread using contact investigation, and (c) replenish the weakened (consumptive) patient to a state of normal weight and well-being. The first two items are performed by public health departments. Clinicians involved in the treatment of TB should verify that the local health department has been notified of all new cases of TB.
Nonpharmacologic interventions aim to (a) prevent the spread of TB, (b) find where TB has already spread using contact investigation, and (c) replenish the weakened (consumptive) patient to a state of normal weight and well-being. The first two items are performed by public health departments. Clinicians involved in the treatment of TB should verify that the local health department has been notified of all new cases of TB.
Workers in hospitals and other institutions must prevent the spread of TB within their facilities.2,11,24 All such workers should learn and follow each institution’s infection control guidelines. This includes using personal protective equipment, including properly fitted respirators, and closing doors to “negative-pressure” rooms. These hospital isolation rooms draw air in from surrounding areas rather than blowing air (and M. tuberculosis) into these surrounding areas. The air from the isolation room may be treated with ultraviolet lights and then vented safely outside. However, these isolation rooms work properly only if the door is closed.
Debilitated TB patients may require therapy for other medical problems, including substance abuse and HIV infection, and some may need nutritional support. Therefore, clinicians involved in substance abuse rehabilitation and nutritional support services should be familiar with the needs of TB patients. Surgery may be needed to remove destroyed lung tissue, space-occupying infected lesions (tuberculomas), and certain extrapulmonary lesions.2,11,37 BCG is the only clinically relevant vaccine for TB in use today. Although it is one of the most commonly administered vaccines in history, it is of limited value, and cannot prevent infection by M. tuberculosis. BCG (discussed below) may prevent extreme forms of TB in infants.37,41
Pharmacologic Therapy
Treating Latent Infection
Isoniazid is the preferred drug for treating LTBI.2,6,11,37 Generally, isoniazid alone is given for 9 months. The treatment of LTBI reduces a person’s lifetime risk of active TB from approximately 10% to approximately 1%. Because TB is spread easily through the air, each case prevented also prevents a second wave of cases that each prevented case would have produced. Historically, the treatment of LTBI has been called prophylaxis. Table 90–2 lists the LTBI treatment options.
TABLE 90-2 Recommended Drug Regimens for Treatment of Latent Tuberculosis (TB) Infection in Adults




