Transthoracic Antireflux Procedures
Tom R. DeMeester
In 1853 Henry Ingersoll Bowditch published a monograph titled A Treatise on Diaphragmatic Hernia in which he commented on the then-current state of knowledge regarding hiatal hernia: “Owing to the ignorance of most of the observers in regard to the true nature of the affection (hiatal hernia), their modes of treatment have been entirely empirical and generally very absurd, and not a few times absolutely harmful to the patient.” Initially, the therapy of hiatal hernia focused only on the surgical correction of the anatomical diaphragmatic defect. The symptoms of hiatal hernia were poorly understood and given no consideration. The possibility that hiatal hernia could encourage the reflux of gastric contents into the esophagus was foreign to the prevailing thought. A typical example of the thinking at the time was one of the publications of Stuart Harrington. He reported on 51 patients with a diaphragmatic hiatal hernia and focused only on describing the anatomical defect and the closure of the hiatus as the surgical treatment. There was no mention of the symptomatology.
It was not until Philip Allison’s publication in 1951 that the symptoms associated with hiatal hernia were linked to the reflux of gastric juice or its contents into the esophagus with the possible development of esophagitis. Allison introduced the term “reflux esophagitis” and emphasized that it was due to a defect in the cardia that allowed acidic gastric juice to reflux into the esophagus. He stated that “it will be noted that it is during inspiration, when the suction pressure in the chest would tend to draw the gastric contents into the esophagus, that the crus of the diaphragm contracts and obstructs this.” Allison theorized, along with others, that “although no internal sphincter can be demonstrated, there is an intrinsic mechanism which contributes to continence.” Allison hypothesized that the latter was due to the anatomical geometry of the gastroesophageal junction since he noted that when the esophagus and stomach were removed from the body, a greater pressure was necessary to drive fluid from the stomach into the esophagus than in the opposite direction. Allison went on to describe the first rational hiatal hernia repair. The focus was on restoring the normal anatomy of the gastroesophageal junction by reducing the hernia and repositioning the gastroesophageal junction into its normal intra-abdominal location. By doing so he hoped to improve the closing of the gastroesophageal junction by the contracting crus, but added that “if the intrinsic mechanism is ineffective, the diaphragm alone may not be enough to ensure complete continence.” Recognition of a high incidence of symptomatic and anatomic recurrence after the repair led Allison to retract the operation in 1973.
Allison’s contribution altered the surgical approach to hiatal hernia disease. Surgeons started to develop procedures designed to place the gastroesophageal junction in an intra-abdominal position in a more effective manner. A posterior gastropexy was devised by Lucious Hill in 1967 which anchored the gastroesophageal junction and its associated phrenoesophageal membrane to the median arcuate ligament of the aortic hiatus. The Nissen full fundoplication, introduced in 1956, and the Belsey Mark IV partial fundoplication introduced by David Skinner 1967 were designed to establish an intra-abdominal segment of esophagus surrounded by a cuff of stomach in the hope of forming a functional flap valve. The Belsey Mark IV repair was performed through a thoracic incision; the Hill repair through an abdominal incision; and the Nissen repair through an abdominal or thoracic incision.
In 1957 Lee Collis added an important contribution to the repair of hiatal hernia. He introduced a method to manage the shortening of the esophagus that occurred with advanced disease due to reflux-induced intramural fibrosis. He described a technique that adds 3 to 4 cm to the esophageal length by creation of a short proximal gastric tube along the lesser curvature of the stomach. This ingenious technique relieved the tension on a hiatal hernia repair caused by a shortened esophagus and reduced the incidence of re-herniation. Later, surgeons added the placement of a partial or full fundoplication around the short gastric tube to improve the competency of the repair.
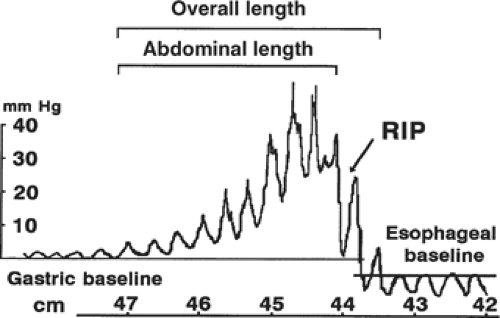
In 1956 esophageal manometry was introduced by F. Earl Fyke and Charles Cole. This technology led to the discovery of a non-anatomical sphincter at the gastroesophageal junction (Fig. 1). This became known as the lower esophageal sphincter and was subsequently shown to be the major barrier against the reflux of gastric contents into the esophagus. In 1979 Dorothy Liebermann-Meffert showed with tedious dissection an asymmetrical muscle thickening in the wall of the esophagus located below the diaphragm and above the angle of His. A manometrically defined high-pressure zone was located in the area and the length of the high-pressure zone was similar to the length of the thickening. The highest pressure was found in the area of greatest muscle thickening. The ability to measure the height and length of the lower esophageal high-pressure zone allowed assessment of various antireflux procedures
in their ability to improve the function of the newly discovered barrier. Of the three procedures, the Nissen full fundoplication was most effective in restoring the competency of the lower esophageal sphincter, followed by the Belsey partial fundoplication and the Hill posterior esophagogastropexy.
in their ability to improve the function of the newly discovered barrier. Of the three procedures, the Nissen full fundoplication was most effective in restoring the competency of the lower esophageal sphincter, followed by the Belsey partial fundoplication and the Hill posterior esophagogastropexy.
In 1991 Bernard Dallemagne performed the first laparoscopic Nissen fundoplication in a human. He successfully performed laparoscopic ligation of the short gastric vessels, safe posterior dissection of the abdominal portion of the esophagus, and plication of the lower esophagus between a fold of anterior and posterior fundus. These were significant accomplishments at that time. Today, laparoscopic Nissen fundoplication has become commonplace, and its safe, effective, and patient-friendly reputation has encouraged its use for advanced reflux disease. The major drawbacks of the procedure are the side effects of postprandial bloating, inability to belch or vomit, and a small but significant incidence of persistent dysphagia.
Today, surgeons are comfortable performing laparoscopically a high mediastinal dissection to mobilize the esophagus and a Collis gastroplasty if at least 2 to 3 cm of distal esophagus does not lie comfortably and free of tension in the abdomen after the mobilization. Currently, only the most complex multiple re-do operation requires an open operation, usually done through a transabdominal incision. Rarely is an open transthoracic Nissen performed. I have not performed one in the past 10 years. The only time a thoracic approach is likely to be considered is when a thoracotomy is required for another reason and an antireflux operation also needs to be done. This being said, it is probably wise to describe the technique just in case the need to perform the procedure arises. Indeed, the well-trained esophageal surgeon should have complete mastery of both a partial and full fundoplication whether performed laparoscopically, transabdominally, or transthoracicly.
Principles of an Antireflux Repair
Antireflux surgery is based on the principle that altering the anatomy of an organ can, in some situations, improve the organs function. For a surgeon to do so requires a thorough knowledge of the organs function and an acceptance that the results, because of the various situations encountered, are likely not to be as good as extirpation surgery. The surgeon’s attention to detail and his ability to adapt the procedure to various situations encountered are essential to achieving the best results. In that regard, there are four principles important in restoring the gastroesophageal barrier that should be understood by all surgeons who perform antireflux procedures.
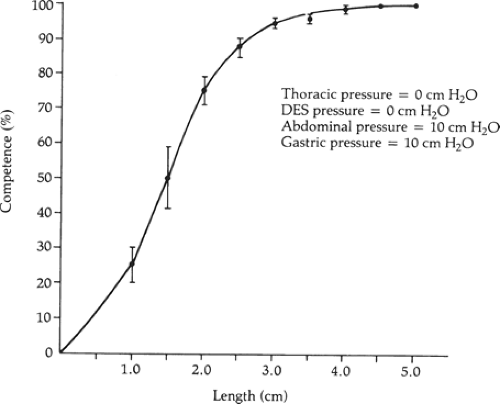
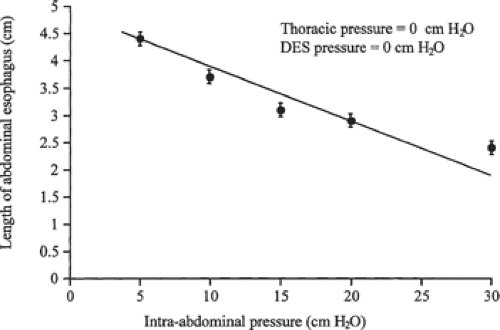
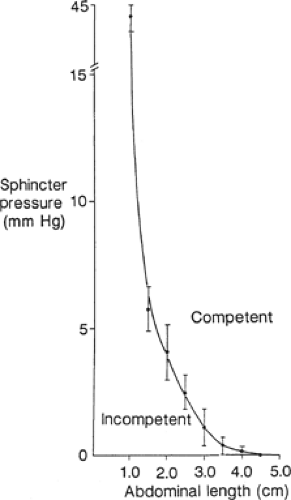
First, the operation should permanently restore at least 2 cm of abdominal esophagus so that the lower esophageal sphincter can respond to the challenges in intra-abdominal pressure. We showed that the degree of competence provided by the lower esophageal sphincter against resting intra-abdominal pressure is a direct function of its abdominal length independent of its intrinsic muscle tone (Fig. 2). This relationship is very efficient in that as intra-abdominal pressure increases, the abdominal length of the lower esophageal sphincter necessary for competence decreases (Fig. 3). The intrinsic muscle tone in the lower esophageal sphincter augments this efficiency further. The relationship breaks down when the abdominal length of the sphincter is ≤1 cm (Fig. 4). At this length even high levels of intrinsic muscle tone are unable to restore competency. Consequently, the permanent restoration of 2 cm of the abdominal sphincter length in a patient with normal sphincter tone maintains the competency of the
sphincter over a wide range of intra-abdominal pressures challenges. These observations encourage sufficient mobilization of the distal esophagus so that a ≥2 cm segment rests below the diaphragm without tension. Further, the construction of a conduit to ensure transmission of intra-abdominal pressure challenges around the abdominal esophagus is also an important aspect of restoring competency. A complete or partial fundoplication serves this purpose. In the absence of a conduit, the development of postoperative periesophageal adhesions can prevent the transmission of intra-abdominal pressure challenges to the abdominal portion of the lower esophageal sphincter. This allows the pressure challenges to act unequally on the stomach and the sphincter’s abdominal length, resulting in sphincter incompetency and reflux.
sphincter over a wide range of intra-abdominal pressures challenges. These observations encourage sufficient mobilization of the distal esophagus so that a ≥2 cm segment rests below the diaphragm without tension. Further, the construction of a conduit to ensure transmission of intra-abdominal pressure challenges around the abdominal esophagus is also an important aspect of restoring competency. A complete or partial fundoplication serves this purpose. In the absence of a conduit, the development of postoperative periesophageal adhesions can prevent the transmission of intra-abdominal pressure challenges to the abdominal portion of the lower esophageal sphincter. This allows the pressure challenges to act unequally on the stomach and the sphincter’s abdominal length, resulting in sphincter incompetency and reflux.
The second principle is to use a technique that prevents reflux from episodes of gastric distention and independent increase in gastric pressure over intra-abdominal pressure. This requires augmenting the resting distal esophageal sphincter pressure to a level equal to or greater than three times the resting gastric pressure (<15 mm Hg), and maintaining an overall sphincter length of at least 2 cm. A gastric fundoplication reliably augments the resting lower esophageal sphincter pressures. The level of increase is related to the degree of fundoplication and is likely due to the addition of gastric muscle tone to the intrinsic muscle tone of the sphincter (Fig. 5).
The transient loss of sphincter length is a recognized cause of reflux in a person with normal sphincter resting pressure and length. Dr. Ayazi has shown that transient losses in abdominal sphincter length are caused by gastric distention. The abdominal sphincter length shortens by effacement into the distended fundus like the neck of a balloon shortens into the distended portion of the balloon with inflation. The shorter the sphincter length becomes, the greater is the intrinsic sphincter pressure required to maintain competence (Fig. 6). A fundoplication acts like a collar around the sphincter that effectively prevents the loss of sphincter length in response to gastric distention.
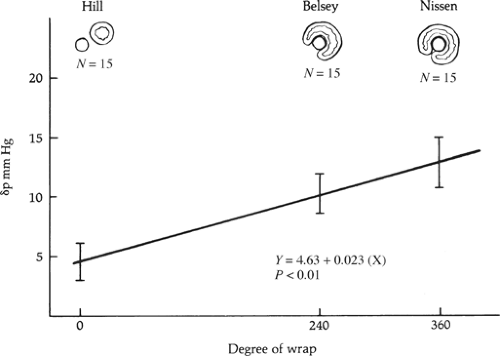 Fig. 5. The increase in postoperative sphincter pressure over preoperative pressure obtained with various degree of fundoplication. |
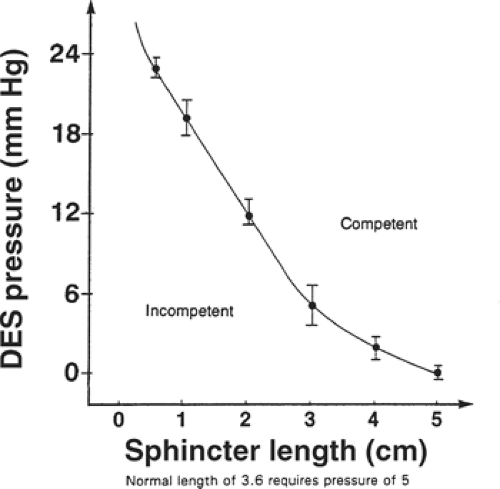 Fig. 6. The relationship of overall lower esophageal sphincter length and pressure to maintain competency against intragastric pressure challenges. DES, distal or lower esophageal sphincter. |
The third principle is to restrict the portion of stomach used to construct the fundoplication to the gastric fundus. To facilitate normal swallowing, the distal esophageal sphincter must relax. On deglutition, a vagal-mediated relaxation of the distal
esophageal sphincter and fundus of the stomach occurs. This relaxation lasts for <10 seconds and is followed by rapid recovery to its former resting tone. To ensure relaxation of the reconstructed sphincter, only the fundus of the stomach should be used to construct the fundoplication since it is known to relax when the distal esophageal sphincter relaxes.
esophageal sphincter and fundus of the stomach occurs. This relaxation lasts for <10 seconds and is followed by rapid recovery to its former resting tone. To ensure relaxation of the reconstructed sphincter, only the fundus of the stomach should be used to construct the fundoplication since it is known to relax when the distal esophageal sphincter relaxes.
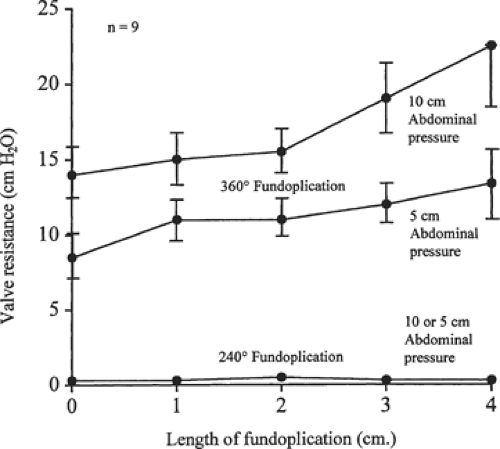
The fourth principle is to use a technique that matches the resistance to flow through the reconstructed sphincter to the propulsive power of the esophageal body. The choice between a 360-degree or partial 240-degree fundoplication is influenced by the strength of the peristaltic contractions. Normal wave progression and strong contractions do well with a complete 360-degree fundoplication. When the prevalence of peristaltic wave progression is reduced to <50%, or esophageal contraction amplitudes in the distal half of the esophagus are ≤20 mm Hg, a partial 270-degree fundoplication is recommended as this degree of fundoplication has no measurable resistance to esophageal emptying (Fig. 7). Inappropriate matching of the body of the esophagus to the resistance of the reconstructed sphincter causes a delay in the passage of food through the sphincter and symptoms of dysphagia. A complete 360-degree fundoplication provides excellent control of reflux but adds considerable resistance to the passage of a bolus through a relaxed sphincter. This resistance is related to the postoperative level of sphincter pressure and the length of the fundoplication. The postoperative sphincter pressure should not be in the hypertensive range and the length of the sphincter should not exceed 1.5 to 2.0 cm (Fig. 7).
Although the indications for an antireflux repair and the biomechanics of an antireflux repair done by laparoscopic and open approaches are similar, there are specific situations in which an open thoracic approach provides an advantage. These include the following:
A patient who has an intrathoracic stomach and a history of multiple previously failed antireflux repairs. A repeat open operation in this situation can be technically easier through the chest.
A patient who has extensive shortening of the esophagus. The thoracic approach in this situation allows for maximum mobilization of the esophagus in order to place the repair, without tension, in the abdomen with or without a gastroplasty to lengthen the esophagus.
A patient with reflux disease who requires a thoracotomy for another reason. If the pulmonary disease is on the left side, an open transthoracic approach allows both problems to be addressed through one incision.
Belsey Mark Iv Partial Fundoplication
Professor Ronald Belsey of Great Britain introduced the Belsey partial fundoplication in 1967. The technical details of the Belsey and the transthoracic Nissen operation are similar, differing only in the completeness of the gastric fundoplication and the length of tension-free intra-abdominal esophagus necessary to perform the repair. The former requires a minimum of 4 cm and the latter 2 cm. Consequently, the clinical success of a Belsey repair decreases progressively from 90% in the patients who have no esophageal shortening to 50% in those who have significant shortening.
The diaphragmatic hiatus is approached through a left posterolateral thoracotomy in the sixth intercostal space (i.e., along the upper border of the seventh rib). For the patients who had a previous failed antireflux repair, we prefer to use the seventh intercostal space. This allows better exposure of the abdomen through a peripheral diaphragmatic incision. The incision is made circumferentially in the anterolateral portion of the diaphragm, 2 to 3 cm from the chest wall, for a distance of 10 to 15 cm. A sufficient fringe of diaphragm must be left along the chest wall to allow for easy closure of the incision. If further abdominal exposure is necessary, the thoracic incision can be extended across the costal margin and diagonally down to the abdominal midline, dividing the fibers of the left rectus muscle. The operation is made easier if a double-lumen endobronchial tube is used for anesthesia and the left lung is selectively deflated.
The esophagus is mobilized from the diaphragm to underneath the aortic arch. Care is taken not to injure the vagal nerves. Branches of the vagal plexus going to the left and right lung must be divided to obtain maximal esophageal length. This allows construction, without tension, of a partial fundoplication over a 4 cm segment of abdominal esophagus. Two vessels arise from the proximal descending thoracic aorta just distal to the arch and pass over the esophagus to the left main stem bronchus. They are the left superior and inferior bronchial arteries. Ligation of these arteries is also necessary to fully mobilize the esophagus. In addition to these arteries, two to three direct esophageal branches come off the distal descending thoracic aorta and pass directly to the esophagus. These are also ligated and divided without concern about ischemic necrosis of the esophagus. There is sufficient blood supply to maintain the integrity of the esophagus through its intrinsic arterial plexus, fed by the inferior thyroid artery in the neck and by the branches from the right bronchial artery in the thorax. This degree of mobilization is necessary to place the repair into the abdomen without undue tension. Failure of adequate mobilization is one of the major causes for subsequent breakdown of a repair and the return of symptoms.
Freeing the gastroesophageal junction and gastric cardia from the diaphragmatic hiatus is the most difficult portion of the procedure, but can usually be completed through the esophageal hiatus. It is unnecessary to make a counterincision through the central tendon of the diaphragm or to enlarge the hiatus by an incision through the crura. The dissection is started by gaining access to the abdominal cavity through the phrenoesophageal membrane. It can be difficult at times to find the correct tissue plane once the membrane has been divided due to the protrusion of the properitoneal fat. Persistence and dissection underneath the retracted left crus, away from the gastric vessels, eventually yield entry into the free peritoneal space. Entering the abdominal cavity is easier when a hiatal hernia is present.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree