Transsternal, Transcervical, and Thoracoscopic Thymectomy for Benign and Malignant Disease Including Radical Mediastinal Dissection
Malcolm M. DeCamp
Alberto De Hoyos
Thymectomy is one of the most common mediastinal procedures performed by thoracic surgeons. Myasthenia gravis (MG), an autoimmune disease characterized by weakness and fatigability following repetitive exercise, is the most common indication for thymectomy. Regardless of the etiology, malignant, benign, or autoimmune, complete resection of all thymic tissue is the key to state-of-the-art thymectomy. Thymectomy can be performed via a variety of operative approaches. The purpose of this chapter is to analyze the pathophysiology of thymic diseases, the indications for surgery in their treatment, and the available techniques of resection to achieve acceptable and durable outcomes.
History of Surgery of the Thymus Gland
The word thymus originates from the Greek word thymos, which has dual meaning. Although it is synonymous with heroic character characterized by soul, spirit, courage, power, will, heart, and anger, it is also related to the herb thyme and to the thyme flower. Rufus (98 to 117 CE), who lived in Ephesus, a center of learning on the western shore of modern-day Turkey, is acknowledged as the first person to refer to the thymus gland in humans.
The thymus gland gained clinical importance once it was linked by the Swiss physician Platter in 1614 to the sudden death of a 5-month-old boy from suffocation. An autopsy was performed at the request of the father as two other sons had died similarly. This revealed a highly vascular mass compressing the mediastinal structures. This condition was subsequently called “mors thymica or thymic death.” More than two centuries later, Sir Astley Cooper in 1832 described the death of a 19-year-old woman from a malignant thymic tumor invading the major veins of superior mediastinum and compressing the trachea.
The finding of thymic tumors in autopsies in 1889 by Oppenheim and in 1901 by Weigert first suggested an interrelation between the thymus and MG. Contemporaneous invention of X-rays by Wilhelm Roentgen in 1895 allowed diagnostic imaging of enlarged mediastinal structures. The first thymectomy for MG was performed in 1911 by Ernst Ferdinand Sauerbruch from Zurich. He used a radiogram to diagnose thymic enlargement, associated it with the patient’s MG symptoms, and performed a successful transcervical thymectomy on a 19-year-old woman.
In 1936, Alfred Blalock became the first person to perform a transsternal thymectomy in a 19-year-old woman with persistent, severe MG and an anterior–superior mediastinal tumor that had only partially responded to three courses of radiotherapy. Beginning with this case, and encouraged by the neurologist Ford, Blalock also demonstrated the relation between MG and nonthymomatous thymus glands.
Sir Geoffrey Keynes followed Blalock in 1942 when he performed a thymectomy and completion thyroidectomy on one of his previous patients with severe MG and recurrent thyrotoxicosis 12 years after removal of a goiter. The subsequent smooth postoperative period encouraged Keynes, who reported a series of 281 thymectomies by 1956. His contributions to the evolution of thymectomy included the separate evaluation of the operative results of patients with thymomatous and nonthymomatous glands, and the precise documentation of surgical thymic anatomy.
The transsternal approach remained the “gold standard” for thymectomy until the early 1960s. After Carlens described mediastinoscopy in 1959, Crile in 1964 and Akakura in 1965 reported their experience with 24 cases of transcervical thymectomy. In addition to MG, the indications for thymectomy were extended to resection of thymic cysts or suppression of rejection in renal transplant recipients. In 1969, Kirschner reported on 21 cases with only 1 death, and suggested that transcervical thymectomy be adopted as the preferred approach to resection, exclusive of thymomas.
Other clinicians remained skeptical regarding completeness of transcervical thymectomy. Reported clinical failures following cervical excisions, in some cases necessitating a reresection, fueled the ongoing debate of the optimal approach to thymectomy for MG. These discussions became more complicated after the reports of Masaoka et al. in 1975 and Jaretzki in 1988 demonstrating the presence of extraglandular thymic tissue in 74% of the cases. Investigators at the University of Pennsylvania have reported comparable results with transcervical thymectomy to transsternal thymectomy after using a specially designed sternal retractor that allowed extended dissection through the limited cervical incision. With this report, the scale again tipped in favor of less-invasive surgery in the debate of thymectomy for MG.
Embryology
The thymus gland, together with the inferior parathyroid glands, arises from the third pair of pharyngeal pouches (Fig. 1). Although the fourth pharyngeal pouches also give rise to a small amount of thymic tissue, these are usually vestigial masses and these latter primordia in humans are usually either absent or rudimentary. In the 6th week of gestation, epithelium of the third pouch proliferates to form a dorsal bulbar component, later becoming the inferior parathyroid gland, and an elongated ventral portion, which is destined to become a thymic lobe. The entire third pouch separates from the pharynx during the 7th week. These thymic primordia migrate in a caudal and medial direction. Originally hollow, thymic primordia rapidly become solid epithelial bars, and during the 8th week, the caudal ends of the paired components of the thymus fuse together to form what is generally a four-lobed gland that attaches to the anterior pericardium. The organ, then being attached to the growing pericardium, descends into the mediastinum behind the sternum and anterior to the great vessels. Incomplete migration may potentially leave islands of thymus anywhere along the course of the primordia. The lower capsule tends to be less distinct, and thymic corpuscles, as well as abundant lymphocytes, trail off into the surrounding mediastinal fat and nodal tissue. Because of either premature arrest of migration or deviation from its natural tract, the thymus gland itself may be found anywhere between the hyoid bone cranially and the xiphoid process of sternum caudally.
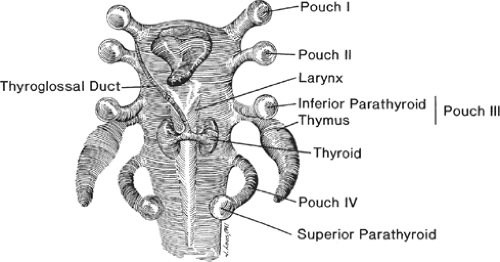 Fig. 1. Embryologic development of the thymus and parathyroid glands from the third and fourth pharyngeal pouches. |
Although the thyroid, parathyroid, and thymus glands arise from the pouches of the same primordial pharynx, they separate in later stages of embryonic life. As the thymus descends caudally, the parathyroid glands normally remain settled at the same level with and posterior to the lower poles of thyroid. The parathyroid glands are also called parathymic glands. In autopsy studies, as many as 20% of inferior parathyroid glands are found within the thymic capsule in the neck or in the mediastinum. Although more common for parathyroid tissue, thyroid tissue may also be present within the thymus gland.
In the newborn, the thymus weighs 10 to 12 g and continues to grow until puberty. Steinmann reported an average weight of 27.3 g with a standard deviation of 16.4 g within the 1st year of life. At puberty, the gland reaches its maximum weight of 20 to 50 g. Thereafter it enters a gradual involution state, decreasing in mass to only 5 to 15 g in the elderly. At this time, the parenchyma is mostly replaced by fibrofatty tissue.
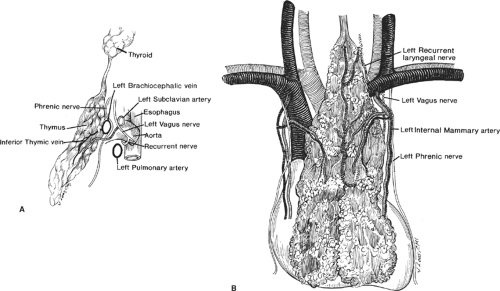
The thymus is a bilobed glandular structure; however, its two lobes are rarely symmetrical. The thymus is located predominantly in the anterior–superior mediastinum, where it anteriorly covers the great vessels, pericardium, and the base of the heart. It is also in close proximity with the anterior surface of the innominate vein as it runs obliquely across the superior mediastinum to join the right brachiocephalic vein to form the superior vena cava (SVC; Fig. 2). The thymus may sometimes lie adjacent to either SVC on the right or the pulmonary artery on the left. One or both lobes may also lie behind the left innominate vein instead of in front of it. The two thymic lobes are fused in the midline, giving the gland its H-shaped configuration. The upper poles of each lobe reach into the neck where they join the thyroid gland by the thyrothymic ligaments on both sides. The lower poles lie on the pericardium anterior to the heart.
The arterial supply of the thymus is chiefly derived from the internal thoracic arteries, although it receives branches from inferior thyroid arteries and
pericardiophrenic arteries as well. There may be small draining veins traveling with the arteries from any of the three sources. However, the main venous drainage is through one or two major trunks, formed by convergence of multiple tributaries from both lobes, running posteriorly between the lobes and draining into the anterior surface of the innominate vein. Although it is a major lymphatic tissue, unlike a lymph node, the thymus gland lacks afferent lymph channels. There are efferent lymph channels, which only drain the capsule and fibrous septa of the gland and drain into anterior mediastinal, pulmonary hilar, and internal mammary lymph nodes. Both sympathetic and parasympathetic nerve fibers are found in the thymus.
pericardiophrenic arteries as well. There may be small draining veins traveling with the arteries from any of the three sources. However, the main venous drainage is through one or two major trunks, formed by convergence of multiple tributaries from both lobes, running posteriorly between the lobes and draining into the anterior surface of the innominate vein. Although it is a major lymphatic tissue, unlike a lymph node, the thymus gland lacks afferent lymph channels. There are efferent lymph channels, which only drain the capsule and fibrous septa of the gland and drain into anterior mediastinal, pulmonary hilar, and internal mammary lymph nodes. Both sympathetic and parasympathetic nerve fibers are found in the thymus.
Numerous islets of thymic tissue, both macroscopic and microscopic, may be found in the neck, middle mediastinum, both pulmonary hili, aortopulmonic window, retrocarinal fat, diaphragm, and even within the pulmonary parenchyma. Masaoka et al. reported 72% of extracapsular microscopic collections of thymic tissue in the anterior mediastinum in 1975, and Fukai et al. came up with the same findings in 51.8% of cases in 1991. Jaretzki documented the variations of extraglandular thymic tissue found in different mediastinal locations in 1997. These publications form the basis for the ongoing debate on completeness and adequacy of thymectomy via various incisions.
Physiology and Pathophysiology
The thymus is a complex organ with epithelial and lymphoid components. During infancy, the thymus is essential for the development of cellular immunity. It is the main site for maturation of null lymphocytes into T cells. The majority of cells in the thymus are T lymphocytes and epithelial cells. These cells comprise the two main structural layers of the gland, the cortex and the medulla, respectively. Occasional lymphoid follicles with B cells and germinal centers can also be found. Although rare, myoid cells may be a part of histologic picture in the thymus. These cells act like skeletal muscle cells, including expression of the acetylcholine receptors (AChRs), and may be involved in the pathophysiology of MG.
The proper function of the immune system depends on the normal simultaneous development of cellular and humoral components, and normal interaction between them. Although thymus-centered T-cell population is responsible for differentiating self-antigens from intruders and producing cell-mediated immune reactions, as seen in delayed hypersensitivity reactions, the bursa-dependent system, formed by the bursa, lymphoid follicles, and plasma cells, is responsible for the production of immunoglobulins (A, G, and M) and specific antibodies. Any disturbance in development of either system may lead to one of the various immunologic deficiency syndromes. Interestingly, some of these syndromes have been associated with various neoplasms of the thymus as well as with congenital thymic hypoplasias and agenesis. Some morphologic changes that the thymus can display have also been linked to hyperplasia, abnormal development, and infections.
Symptoms related to thymic disease are categorized as the ones arising directly from the thymic lesion by either compression or invasion, symptoms associated with a previously described clinical syndrome (i.e.,
MG, red cell aplasia, or hypogammaglobulinemia), or nonspecific systemic symptoms such as anorexia and fatigue. Developmental anomalies can involve the location of the thymus or its development. Failure of the thymus to descend into the anterior mediastinum might account for cervical thymic tissue, which can be mistaken for neoplasm, lymphadenopathy, or an enlarged parathyroid. This aberrant tissue can cause compressive symptoms such as stridor or dysphagia. Lymphocyte count and tests of immunologic capacity may be diminished by thymectomy; however, no particular clinical problems have developed to correlate with these laboratory observations.
MG, red cell aplasia, or hypogammaglobulinemia), or nonspecific systemic symptoms such as anorexia and fatigue. Developmental anomalies can involve the location of the thymus or its development. Failure of the thymus to descend into the anterior mediastinum might account for cervical thymic tissue, which can be mistaken for neoplasm, lymphadenopathy, or an enlarged parathyroid. This aberrant tissue can cause compressive symptoms such as stridor or dysphagia. Lymphocyte count and tests of immunologic capacity may be diminished by thymectomy; however, no particular clinical problems have developed to correlate with these laboratory observations.
Thymic Neoplasms
The epithelial subset of thymic neoplasms, namely, thymoma, is the most common tumor of the anterior mediastinum. It is fundamental in oncology that a successful therapeutic approach to a neoplasm be based on a definitive and prognostically reproducible classification and staging system. However, the classification of thymic tumors has been one of the most debated subjects of modern thoracic surgery and oncology. There have been several classification proposals, focusing on one or more of histology, embryology, and biology of the tumors, in an attempt to establish an agreed-on nomenclature. Although lymphomas are far more common than thymomas in children and adolescents, the only role for surgery in their treatment is for diagnostic and staging purposes. Mesenchymal and germ cell tumors of the thymus are uncommon. The most common type is teratoma. Seminomas and nonseminomatous germ cell tumors also occur in the thymus, almost exclusively in men. Thymic carcinoids are extremely rare, often associated with endocrinopathies such as Cushing syndrome or inappropriate secretion of the antidiuretic hormone. They are typically invasive, often recur, are associated with extrathoracic metastases, and have a poor prognosis. The thymus can rarely be a site for metastasis as well.
Thymoma
Thymomas are rare tumors. Despite this, they account for 20% of all mediastinal neoplasms and comprise half of all primary tumors found in the anterior compartment. They may also be encountered in other areas of the neck and the thorax, such as, but not limited to, along the pleura, inside the pericardial sac, over the diaphragm, within the lung parenchyma, and even as a polypoid lesion in the trachea. Thymomas are slow-growing tumors; however, they exhibit malignant potential as some demonstrate local invasion, pleural dissemination, and systemic metastases without overt cytologic features of malignancy. They are more common between ages 40 to 60 and do not show predilection for either sex.
The clinical presentation of thymoma is extremely varied. In up to 50% of instances, these neoplasms are entirely asymptomatic, discovered incidentally on chest imaging or at autopsy. Approximately 30% of patients present with local symptoms related to pressure or direct invasion. In 20% to 70% of patients, thymoma is associated with systemic disorders that are primarily of autoimmune origin. When present, these concomitant diseases worsen the prognosis. The most common autoimmune disease associated with thymoma is MG. However, up to 28% of thymoma patients will present with an immune disorder other than MG. The most common disorders include pure red cell aplasia, lupus erythematosus, polymyositis, and hypogammaglobulinemia.
All thymomas originate from the thymic epithelial cells; however, only about 4% of them consist of a pure population of epithelial cells. Most have mixed populations of lymphoid cells, to a varying extent. There have been two major approaches to histologic classification of thymomas. In 1961, Bernatz et al., from Mayo Clinic, divided thymomas into lymphocytic, epithelial, mixed, and spindle subtypes according to the lymphocyte-to-epithelial cell ratio in tumors. At that time, thymic carcinomas were not distinctly segregated, but grouped with thymomas. In 1978, Levine and Rosai proposed a new classification, which proved to be of high clinical relevance. In this report, they divided the thymomas into benign (circumscribed) and malignant (invasive) types. Malignant thymomas were further divided into type I (invasive thymoma with minimal atypia) and type II (showing moderate to marked atypia). In this system, type II malignant thymomas correspond to thymic carcinomas. Wick et al., in 1982, and Lewis et al., in 1987, proposed the separation of thymic epithelial cell neoplasms into thymomas and thymic carcinomas, which is now well accepted. Mixed thymoma cases with islets of thymic carcinoma behave clinically like typical thymomas more than like thymic carcinomas. These findings support the theory that thymomas carry the potential for malignant transformation into malignant thymic carcinoma.
The Bernatz system remained the mainstay of thymoma classification until the mid-1980s. In 1985, Marino and Müller-Hermelink proposed a classification system based on the origin of the cells more than the cell type (i.e., cortical, medullary, or mixed subtypes). They differentiated the origin of epithelial cells found in the thymoma according to their resemblance to the normal epithelial cells in other parts of the thymic lobule and classified the tumor accordingly. In cortical thymomas, the epithelial cells are large and round or polygonal, with clear round nuclei, conspicuous nucleoli, and poorly defined cytoplasm. In contrast, the epithelial cells of medullary thymomas are smaller and spindle-shaped with irregular or fusiform nuclei and inconspicuous nucleoli. The histologic features of cortical thymomas suggest a more malignant phenotype, and indeed they are more aggressive. This classification was suggested to have independent prognostic implications. In their 80-patient series in 1990, Pescarmona and colleagues found that the Müller-Hermelink (M-H) classification reliably predicted prognosis. Medullary thymomas tended to be more encapsulated and clinically acted benign, but cortical ones were invasive and malignant in nature. When a modification of the Bernatz classification was used in the same 80-patient series, there was no correlation found between the subtypes and their prognosis. Wilkins (1995) also confirmed the M-H results, as he noted only a few recurrences in patients with medullary or mixed tumors, whereas the recurrence rate was much higher in cortical tumors.
The World Health Organization’s (WHO) “Histological Typing of Tumors of the Thymus,” reported by Rosai in 1999, reflected the consensus of the pathologists specializing in thymic tumors (Table 1). The cellular origins of the various neoplasms are emphasized in this classification, which is a successful synthesis of the most widely used classifications and resembles more the M-H classification. Currently, the WHO classification appears to be the preferred classification method in thymic neoplasms. In this classification, type A represents atrophic adult-life thymic cells that are spindle or oval in shape, and type B represents bioactive thymic cells, of fetus and infants, with dendritic or epithelioid appearance. Type B thymomas are further divided into B1, B2, and B3 on the basis of increasing epithelial-to-lymphoid ratio and the emergence of atypia of the epithelial cells. Type AB thymomas display the common features of type A and type B lesions. Type C tumors are frankly malignant cells, and these lesions correspond to thymic carcinomas. There are at least nine subtypes of type C tumors, and they may be further subdivided
into low-grade and high-grade malignant tumors.
into low-grade and high-grade malignant tumors.
Table 1 World Health Organization (WHO) Classification of Thymomas and Carcinomas of the Thymus | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Besides the efforts of classifying the thymomas according to their histologic or morphologic features, Masaoka et al. (1981) proposed an anatomic classification based on the presence or absence of gross or microscopic invasion of the tumor capsule as well as its metastatic status (Table 2). Medullary and mixed histology tumors are usually not invasive and therefore correspond to Masaoka stages I or II, whereas cortical thymomas are more commonly invasive and more likely to be Masaoka stages of III or IV. Later modifications of the Masaoka classification, as suggested by Koga et al. (1994) and Nakagawa et al. (2003), have been more widely adopted (Table 3). These incorporated microscopic incomplete capsular invasion into stage I, leaving only transcapsular invasion in stage II.
Table 2 Masaoka Clinical Staging of Thymomas | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
As part of the WHO thymoma classification effort, it was suggested that a TNM scheme be used in staging of all malignant thymomas and thymic carcinomas (Table 4). Because there has been so much controversy regarding tumor classification during the past four decades, no authorized TNM system has been adopted. The proposed WHO TNM schema remains tentative, pending validation of its reliability, reproducibility, and predictive power.
To further enhance our understanding of thymic malignancies and provide support to patients and families with thymic cancer, the Foundation for Thymic Cancer Research (FTCR) organized meetings of international leaders in the field, resulting in collaborative initiatives. These include several published meta-analyses as well as National Comprehensive Cancer Network (NCCN) guidelines for the management of thymic malignancies. The FTCR partnered with the National Cancer Institute to organize the first International Conference on Thymic Malignancies in 2009 (www.thymic.org). As a result of these efforts, the International Thymic Malignancy Interest Group (www.itmig.org) was created with broad international and multidisciplinary representation and held its inaugural meeting in May 2010.
Table 3 Modified Masaoka Clinical Staging of Thymomasa | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||
Myasthenia Gravis
The original description of MG dates back to the case report of the physiologist Sir Thomas Willis in 1672. The real recognition of the clinical syndrome came after the report by Wilks in 1877, in which he described a young woman who died of a respiratory paralysis. Autopsy did not reveal any central nervous system pathology that would explain the problem. In 1895, Jolly described the test now bearing his name, disclosing the easy fatigability in the affected muscle following repetitive stimuli. He then proposed the name myasthenia gravis pseudoparalytica for the disease. In 1899, the Berlin Society for Neurology and Psychiatry shortened the name to myasthenia gravis. However, only during the last two decades has the pathophysiology of this disorder become well elucidated as the experiments revealed the microstructure, physiology, and molecular composition of the nicotinic AChR.
The prevalence of MG has been estimated at 43 to 64 per million population. It has a predilection for females with a ratio of 3:2. Its peak age is 20 to 30 in women and more than 50 years in men. The disease is usually nonfamilial. The common presenting symptom of the disease is an insidious onset of generalized weakness. This is seen in 85% of the patients. It is usually symmetrical and becomes more intense at the end of the day. Muscles innervated by cranial nerves are often the first to be affected; however, any striated muscle in the body may be involved. Some patients may present with external ocular symptoms only, such as ptosis and diplopia. These symptoms may either spread to other muscle groups or remain confined to the eye, and may be so subtle that patients may go through several eyeglass prescriptions before they are diagnosed with MG. The worst type of disease occurs when the bulbar muscles are involved. The symptoms may include dysarthria with nasal tones caused
by palatal paresis, diminished voice, and dysphonia. Often, patients can bite on the food but as they continue to eat, the mastication muscles weaken until they can no longer chew or close their jaws. Dysphagia may be associated with nasal regurgitation of liquids. Weakening of neck and back muscles may necessitate manual support of the head. Pelvic weakness may result in waddling gait. The most dreaded complication, however, is respiratory involvement. Weakness of respiratory muscles with paresis of intercostal muscles and the diaphragm may necessitate emergent medical attention. The Osserman and Genkins classification of MG is still very useful and widely adopted (Table 5). Standardized preoperative clinical staging has been recommended by applying the Myasthenia Gravis Foundation of America (MGFA) clinical classification (Table 6). Postoperative assessment can be monitored again in a standardized schema by following the De Filippi classification (Table 7).
by palatal paresis, diminished voice, and dysphonia. Often, patients can bite on the food but as they continue to eat, the mastication muscles weaken until they can no longer chew or close their jaws. Dysphagia may be associated with nasal regurgitation of liquids. Weakening of neck and back muscles may necessitate manual support of the head. Pelvic weakness may result in waddling gait. The most dreaded complication, however, is respiratory involvement. Weakness of respiratory muscles with paresis of intercostal muscles and the diaphragm may necessitate emergent medical attention. The Osserman and Genkins classification of MG is still very useful and widely adopted (Table 5). Standardized preoperative clinical staging has been recommended by applying the Myasthenia Gravis Foundation of America (MGFA) clinical classification (Table 6). Postoperative assessment can be monitored again in a standardized schema by following the De Filippi classification (Table 7).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree