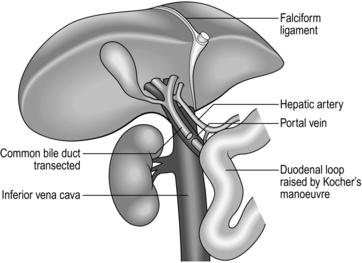
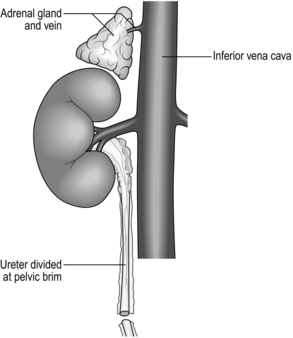
26 1. Solid whole-organ transplantation has been one of the main events in the evolution of 20th and 21st-century medicine. A kidney transplant offers a quality of life unattainable by long-term dialysis, and the lack of long-term artificial support for end-stage disease of the liver, heart and lungs makes it likely that there will be a demand for organ transplantation into the foreseeable future. 2. Immunosuppressive agents that reduce or abolish graft rejection are vital to the success of organ transplantation. Ciclosporin, acting by calcineurin inhibition within T-cell lymphocytes, blocks progression of the cell cycle from G0 to G1 and the production of interleukin 2. In the early 1980s, when used alone or with steroids it improved the 2-year graft survival following kidney transplantation from 50% to 75–80%. Newer drugs include tacrolimus, rapamycin and mycophenolate mofetil. Other interesting molecules differing in their mode of action are leflunomide, brequinar, mizoribine, deoxyspergualin. Brain-stem dead, heart-beating ‘cadavers’ – over 80% of solid-organ donors, usually providing multiple organs. Non-heart-beating cadavers, providing suitable organs for kidney and increasingly for liver transplantation, occasionally lung transplants but not for hearts. Living related donors, such as identical twins, siblings, parents, children, first-order cousins, providing excellent donor organs for kidney transplants; and, with appropriate techniques, segments of livers, pancreas and lung can be grafted. Living unrelated donors: spouses, partners, friends, altruists and paid donors (the latter illegal in the UK). 4. The success of organ transplantation has resulted in a shortage of solid organs for transplantation. There are almost 8000 patients waiting in the UK and 70 000 in the USA. The shortfall results in part from cultural, religious, financial, legal and political conditions and varies in different countries. In addition, permission to remove organs after death is usually determined by prior consent of the donor or of the surviving relatives. In some countries, although not in the UK, potential donors are required to ‘opt out’ – that is, state while alive that they refuse to have organs removed – otherwise it is assumed that they permit it. In the UK ‘opt out’ legislation is currently being debated. Other discussions taking place include the practicalities and ethics of xenotransplantation (Greek: xenos = strange, foreign) – the use of animal organs – and cloning (using nuclear replacement technology) as a means of providing auto-transplantable tissues. 5. Brain-stem death must be established before organs are removed from a mechanically ventilated patient with irreversible cerebral destruction. If the plasma electrolytes or blood gases are abnormal, or if there is suspicion of drug intoxication, organ donation is not pursued. The diagnosis must be made by qualified medical practitioners independent of the transplant team, carrying out the examination individually on two separate occasions. The following signs must be absent: 6. Tissue transplantation has been successfully practised for many years, including the cornea, which as a ‘privileged site’ evokes no rejection and requires no immunosuppression. Bone and skin are also transplanted, but essentially serve to provide a non-cellular matrix into which recipient cells can grow as the donor cells are destroyed. Immunosuppressive agents are again unnecessary. 1. Exclude potential donors if there is any possibility of transmitting to potential recipients the following: 2. Scrutinize the social history of the donor as far as possible. Exclude prospective donors with a clear history of intravenous drug abuse, prostitution or homosexuality. 1. The ventilated heart-beating brain-dead donor may be physiologically unstable, requiring inotropes to maintain systemic vascular resistance and other pharmacological agents such as pitressin or desmopressin (des-amino-des-aspartate-arginine vasopressin, DDAVP) to control diabetes insipidus. Carefully maintain fluid balance, so be willing to institute invasive monitoring such as Swan-Ganz catheterization and direct arterial pressure measurements. Pulse oximetry and cardiac monitoring are essential. Have available body-warming equipment to compensate for hypothermia. 2. Have an anaesthetist in attendance. A general anaesthetic is unnecessary, but give a neuromuscular blocking agents such as curare before making the first incision, to prevent muscular spasms and spinal reflexes, which may be induced by the surgical procedure. 3. Blood loss during the surgical procedure may profoundly destabilize the donor’s cardiovascular status and you must therefore take great care to minimize blood loss at all times. Have available 4–6 units of cross-matched bank blood for use during the surgical procedure. 4. The donor surgical team must be self-sufficient and provide surgical instruments, cannulae, sterile bags for the excised organs, ice, cooling fluids, preservation fluids and cardioplegic solution. 1. Make a midline incision from the jugular notch to the symphysis pubis. 2. Split the sternum longitudinally with a Gigli saw. Leave the pericardium and pleural cavities intact if possible. 3. Retract the sternal edges with a self-retaining retractor after securing haemostasis by liberal application of bone wax. 1. Perform a detailed inspection of all abdominal contents to exclude unsuspected pathology, the incidence of which increases in proportion to age. Absolute contraindications to proceeding with organ retrieval are peritoneal contamination due to ruptured bowel and the presence of disseminated intra-peritoneal cancer. Cirrhosis of the donor liver precludes transplantation of that organ. 2. Focal abnormalities found in one or more sites in the abdomen or chest need not prohibit organ retrieval, but remove biopsy specimens of all suspicious lesions for histological examination. You must not re-implant retrieved organs before excluding malignancy histologically. 1. Commence careful dissection of the structures in the free edge of the lesser omentum leading to the porta hepatis. Ligate and divide the common bile duct just above the duodenum. Dissect and control the portal vein with a rubber sling. Carefully search for abnormalities of the hepatic arterial supply. Seventeen per cent of donors have an accessory or aberrant right hepatic artery passing to the porta hepatis posterior to the portal vein and common bile duct. Twenty-three per cent have an accessory left hepatic artery arising from the left gastric artery. Identify these variations early and preserve the vessels.1 2. Mobilize the duodenum by Kocher’s manoeuvre. Isolate the inferior vena cava above the renal veins and below the liver, and control it with a nylon tape. 3. Divide the peritoneal attachments of the liver, starting with the left triangular ligament and proceeding to the falciform ligament, thus exposing the anterior surface of the suprahepatic vena cava (Fig. 26.1). 4. Divide the right triangular ligament and continue by dividing the upper and lower layers of the coronary ligament while progressively dislocating the liver upwards and to the left, thus separating the liver from the bare area of the diaphragm. Ligate and divide the right adrenal vein, which has now been exposed, and continue dissecting to free the retrohepatic vena cava from the posterior abdominal wall. Achieve complete mobilization of the vena cava by dividing the peritoneum of the right side of the lesser sac. Divide the remnant of the lesser omentum, leaving the liver attached by its blood vessels only. 5. Begin mobilization of the right kidney by dissecting the peritoneum from its anterior surface and controlling the right renal vein and vena cava below the renal vein with rubber slings. 6. Gently lift the kidney from the renal fossa and sweep away surrounding fatty tissue to expose the renal artery or arteries and vein, and control them with further rubber slings. 7. Dissect the ureter with plenty of surrounding connecting tissue down as far as the pelvic rim and then divide it (Fig. 26.2). 8. Repeat the procedure for the left kidney. In addition, ligate and divide the left adrenal and gonadal veins. Also identify, ligate and divide the large lumbar vein opening into the posterior aspect of the left renal vein. 9. Expose and control the lower aorta just above the bifurcation, for subsequent cannulation and flush-cooling of the liver and kidneys. 10. Dissect and control the superior mesenteric vein below the transverse mesocolon for later cannulation of the portal vein and flush-cooling of the liver. 11. Mobilize the pancreas at this stage if it is required. Completely divide the gastrocolic and gastrosplenic omentum. Retract the stomach to expose the pancreas. Divide the lienorenal ligament and, using the spleen as a ‘handle’, carefully mobilize the tail and the body of the pancreas, working towards the midline. Dissect and control with a rubber sling the origin of the splenic artery at the coeliac axis. 12. The cardiac surgical team should now prepare the required thoracic organs for removal. When all the dissections have been completed give 30 000 IU of heparin intravenously. Place a cannula (size 16–20 F) in the portal vein via the superior mesenteric vein and place another in the infrarenal abdominal aorta. Place a third cannula in the infrarenal vena cava. 13. Stop mechanical ventilation. Stop circulation with cardioplegic solution infused through the aortic root. Start flush-cooling of abdominal organs via the portal and aortic cannulas using 3–6 L of University of Wisconsin solution (Viaspan) at 4 °C. Simultaneously exsanguinate the donor via the inferior vena caval cannula to ensure venous decompression and thus effective flush-cooling of the abdominal organs. 14. Following removal of the thoracic organs by the cardiac surgical team wait until the abdominal organs are visibly pale and palpably cold before removing them by dividing the remaining vascular connections. Follow the coeliac axis and the renal arteries back to the aorta. In each case excise a rim of aorta – a ‘Carrel patch’. 15. Remove all cannulas. Suck out all free fluid and make a careful watertight wound closure using continuous 0 or no. 1 nylon. 1. Cool the organs in situ by infusing large volumes of isotonic cold crystalloid solution such as Viaspan, thus reducing the temperature of the perfused organs to 8–15 °C. 2. Excise the organs when they are visibly pale and palpably cold. Flush them through once more with approximately 1 L of a preservation solution (University of Wisconsin solution for the liver and Marshall’s hypertonic citrate solution for the kidneys) which will equilibrate throughout the extracellular space of the organ during the preservation period. 3. Double-wrap the organs in sterile bags and place them in boxes of ice where they will remain until re-implantation. Cooling continues to approximately 0 °C in ice over the next few hours. 4. Currently used preservation solutions are hypertonic, contain non-diffusible large anions and usually have a high potassium content corresponding with that of intracellular fluid. These solutions are more effective than physiological saline for cold preservation of organs because they prevent cell swelling and intracellular electrolyte loss during the hypothermic inactivation of the sodium pump. 5. Immediate life-supporting function can be expected from a kidney that has been preserved in one of these solutions for up to 24 hours. Between 24 and 72 hours viability will be preserved but, because of acute tubular necrosis, delayed function is likely. 6. Immediate life-supporting function is an absolute prerequisite for the transplanted liver or heart, thus reducing the safe preservation time dramatically. For the liver, University of Wisconsin solution is clearly the best preservation solution, allowing preservation time for up to 20 hours. For the heart, 4–6 hours is currently the safe limit. 7. Record on the forms provided the donor’s demographic details, blood group, anatomical abnormalities and time of circulatory arrest. Record the type and volume of preservation solution used. Arrange for samples of donor spleen and lymph node to accompany each organ, together with the copy of the data form, to its final destination. 1. Despite 30 years of clinical organ transplantation, the role of human leucocyte antigen (HLA) matching remains controversial and enigmatic. At least four different gene loci on chromosome 6 code for the human major histocompatibility complex (MHC) and are known as HLA A, B, C and D. Currently, antigens that are cell-surface gene products, usually glycoproteins, of the A B and D loci are routinely determined and matched for donor and recipients in renal transplantation. 2. For renal transplantation from closely related donors such as a parent or sibling, graft survival appears to be directly related to the degree of HLA matching, whereas in the unrelated cadaver donor situation, kidneys fully matched at the A, B and D loci (six antigens) enjoy outstandingly good survival, but any lesser degree of matching significantly prejudices survival. 3. In liver transplantation and heart transplantation no convincing relationship has yet been demonstrated between HLA matching and graft survival. Donors and recipients are usually paired on the basis of ABO blood group compatibility only. 1. Donor kidneys for transplantation may be obtained from: 2. Kidney transplantation currently offers the best chance of long-term survival combined with near-normal quality of life for those suffering from end-stage chronic renal disease. 3. The cost of a well-functioning kidney graft, or any other organ graft, is lifelong treatment with non-specific immunosuppressive agents. Ultimately, the main threat to long-term survival of the kidney graft recipient is likely to be the complications of long-term immunosuppressive therapy in the form of infections and an increased incidence of neoplasia. In addition, hypertension and accelerated cardiovascular disease are commonly seen. After 5 years, recipient death with a functioning graft is the commonest cause of graft loss. 4. End-stage renal disease of all types comprises the indications for renal transplantation. Chronic glomerulonephritis accounts for 60% of all cases of chronic renal failure. Diabetic nephropathy, refluxing pyelonephritis, polycystic disease and previously failed kidney grafts are the other main indications for transplantation. 1. Perform HLA tissue typing and ABO blood grouping. Ensure compatibility with the recipient. Screen for hepatitis B and C and HIV. Demonstrate proof of genetic relationship between donor and recipient by DNA fingerprinting (restriction fragment length polymorphism). 2. Perform diethylenetriamine pentaacetic acid (DTPA) scan to ensure that each of the potential donor’s kidneys contributes roughly 50% to total renal function. Gross functional asymmetry between the two kidneys will prohibit donation. 3. Perform an aortogram with selective views of both renal arteries, or magnetic resonance angiography. A kidney with a single renal artery is desirable. Multiple renal arteries supplying both kidneys prohibit kidney donation. 4. Ensure that kidney preservation solutions, ice, organ bags, and cannulae are available for the operative procedure. 1. Place the anaesthetized donor on the operating table in the lateral position. 2. Make a 20-cm muscle-cutting loin incision over the 12th rib. Gain access to the retroperitoneal space through the bed of the 12th rib. Take care to avoid the pleura, which is attached to the medial half of the upper border of the 12th rib. Sweep the peritoneum forward, keeping it intact. 1. Carefully dissect the kidney free of its perirenal fat and the adrenal gland and gently draw it up into the wound to facilitate dissection of the renal pedicles. 2. Dissect both renal vein and renal artery and control each with a soft rubber sling. On the left side divide and ligate the gonadal vein and the adrenal vein. Look for a large lumbar vein entering the posterior aspect of the renal vein. Ligate and divide it. Mobilize the renal vein fully to its confluence with the inferior vena cava. 3. Dissect the renal artery to the aorta. On the right side this requires elevation and retraction of the inferior vena cava and necessitates ligation and division of one or more lumbar veins. 4. Dissect the ureter to the pelvic brim. Ligate it distally at this point and divide it. 5. When attached by its blood vessels only, confirm that the kidney is undergoing diuresis. Give 30 g of mannitol intravenously. Apply vascular clamps to the renal vessels and rapidly excise the kidney. Take it to a side trolley and rapidly flush-cool via the renal artery using a kidney preservation solution at 4 °C. Continue perfusion of the kidney until the effluent from the renal vein is clear and the kidney is palpably cold. 6. Double-wrap the kidney in sterile polythene bags and place it in a bag of ice until the time for reimplantation. 7. Carefully ligate the cut donor renal vessels and close the loin incision in layers, inserting a silicone tube drain.
Transplantation
INTRODUCTION
 Spontaneous breathing when disconnected from the ventilator under hypercarbic conditions monitored by blood gases.
Spontaneous breathing when disconnected from the ventilator under hypercarbic conditions monitored by blood gases.
THE MULTIPLE ORGAN DONOR
Appraise
Donor screening and exclusions
Prepare
Access
Assess
Action
ORGAN PRESERVATION
TISSUE TYPING
KIDNEY TRANSPLANTATION
Appraise
 Living related donors – usually confined to those with close genetic links such as mothers and fathers, sisters and brothers and, occasionally, first-order cousins. The results of living related kidney transplantation have always been superior to those of cadaver kidney transplantation. In the UK, the Human Organ Transplant Act 1989 stipulates that for any proposed living related organ donation genetic relationship between the donor and the recipient must be established by DNA fingerprinting before the transplant can legally proceed.
Living related donors – usually confined to those with close genetic links such as mothers and fathers, sisters and brothers and, occasionally, first-order cousins. The results of living related kidney transplantation have always been superior to those of cadaver kidney transplantation. In the UK, the Human Organ Transplant Act 1989 stipulates that for any proposed living related organ donation genetic relationship between the donor and the recipient must be established by DNA fingerprinting before the transplant can legally proceed.
 Living unrelated donors – a small but increasing number of transplants have been performed between related, but not genetically linked, individuals such as husbands and wives and vice versa. Close friends may also donate. Before a living unrelated donation can legally take place in the UK, a dispensation from the Human Tissue Authority must be obtained, and is a statutory requirement.
Living unrelated donors – a small but increasing number of transplants have been performed between related, but not genetically linked, individuals such as husbands and wives and vice versa. Close friends may also donate. Before a living unrelated donation can legally take place in the UK, a dispensation from the Human Tissue Authority must be obtained, and is a statutory requirement.
 Unrelated brain-stem-dead heart-beating cadaver donors – organs are removed immediately following arrest of the circulation. Eighty per cent of all kidney transplants occurring in the UK are based on this type of donor.
Unrelated brain-stem-dead heart-beating cadaver donors – organs are removed immediately following arrest of the circulation. Eighty per cent of all kidney transplants occurring in the UK are based on this type of donor.
 Unrelated non-heart-beating cadaver donors – kidneys may be used from a donor following a cardiac standstill as long as the kidneys can be safely removed and cooled within 60 minutes of circulatory arrest. This type of donation is often associated with delayed initial function of the transplant kidney due to acute tubular necrosis.
Unrelated non-heart-beating cadaver donors – kidneys may be used from a donor following a cardiac standstill as long as the kidneys can be safely removed and cooled within 60 minutes of circulatory arrest. This type of donation is often associated with delayed initial function of the transplant kidney due to acute tubular necrosis.
THE OPEN APPROACH
Prepare
Access
Action
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Transplantation