Chapter 11 Lung transplantation
Human lung transplantation was first performed in 1963 but few patients survived and the operation was largely abandoned until the advent of ciclosporin and the development of combined heart and lung transplantation nearly 20 years later. Today over 30 000 lung transplants have been performed and the procedure is firmly established amongst the therapeutic options available for patients with severe lung disease.1 Donor shortage is the main factor limiting its application and this drives the search for new strategies to increase the number of lungs available for transplantation. These include the use of live donors, easing of donor selection criteria and ex vivo techniques to recondition lungs of marginal viability.2,3About 2% of lung transplants are repeat operations, performed because of graft failure. Patients receiving lung transplants have ranged from infants to the elderly, with the peak age being about 50 years. The principal conditions treated by lung transplantation in adults have been chronic obstructive pulmonary disease/emphysema (36%), idiopathic pulmonary fibrosis (21%), cystic fibrosis (16%), α1-antitrypsin deficiency (7%) and primary pulmonary hypertension (4%). The remainder include sarcoidosis, lymphangioleiomyomatosis, connective tissue disease and, rarely, lung cancer.4 The commonest indication for lung transplantation in adolescents is cystic fibrosis and in children congenital heart disease.5
Types of lung transplant
Combined heart and lung transplantation, which was first performed in 1981, was followed by single-lung transplantation, then double-lung transplantation, and lastly sequential bilateral lung transplantation. The combined operation requires total cardiopulmonary bypass and if successful carries a risk of accelerated coronary atheroma and problems resulting from cardiac denervation. However, it is relatively simple technically, maintains coronary–tracheobronchial arterial anastomoses that help the tracheal anastomosis to heal, and is particularly suitable when both heart and lungs are damaged, as in pulmonary hypertension. In cystic fibrosis, it is necessary to replace both lungs to avoid the risk of spillover infection. Double-lung transplantation is a complex procedure but was initially used in emphysema because it was feared that with single-lung transplantation the native diseased lung would be preferentially ventilated. This proved not to be the case and single-lung transplantation is now widely used for both severe emphysema and pulmonary fibrosis. It is the commonest procedure, the simplest to perform, is associated with the fewest postoperative complications, requires the least amount of donor tissue and enables the greatest number of recipients to benefit from a single donor.6
Except for bronchial artery revascularisation, which is undertaken in only a few centres, no attempt is made to reanastomose the severed tracheal or bronchial blood vessels and nerves in any of these operations, or the lymphatics, which are also severed if the heart is not included. Loss of these structures promotes postoperative haemorrhage, breakdown of the tracheal or bronchial anastomosis, a reduction in the cough reflex and pulmonary oedema. A further aspect of lung transplantation is that some lymphatic tissue is inevitably included in the allograft, entailing a risk of graft-versus-host disease. This is greatest when the whole mediastinum is transferred, as in combined heart and lung transplantation, but in practice it is a rare complication.
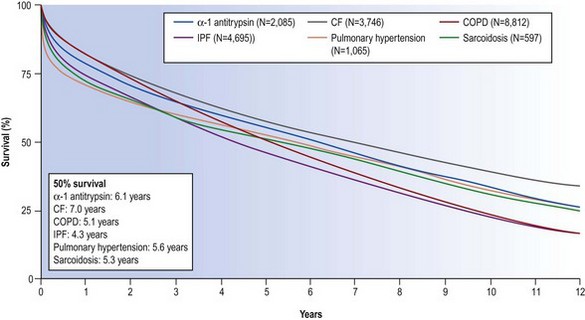
The mortality associated with lung transplantation is constantly diminishing as techniques and immunosuppression improve. In 2009 the International Society of Heart–Lung Transplantation reported survival rates of 79%, 52% and 29% at 1, 5 and 10 years respectively for lung transplantation and 64%, 41% and 26% at the same periods for combined heart–lung transplantation (Fig. 11.1).4 In the first postoperative month mortality is chiefly due to sepsis, haemorrhage and poor lung preservation. After the first month the principal causes of death are infection and rejection in the form of obliterative bronchiolitis.
Recipient selection
Lung transplantation is an operation of last resort. There are insufficient donors and patients are unlikely to be considered unless other measures have failed and their short-term prognosis is otherwise poor. The presence of uncontrolled systemic disease precludes consideration and good renal and hepatic function is essential, particularly in view of immunosuppressant drug toxicity. This is particularly important in α1-antitrypsin deficiency and cystic fibrosis, both of which may affect the liver directly. Any infection that cannot be eliminated, either before or by the operation, is likely to disseminate postoperatively because of the immunosuppression and therefore militates against successful transplantation. An aspergilloma is a contraindication to any form of lung transplantation as its attempted removal inevitably leads to seeding of the pleural cavity and mediastinum. Previous thoracic surgery may make it difficult to operate and the possibility of a future lung transplant should therefore be borne in mind when considering the best treatment for conditions such as pneumothorax. Left ventricular function should be normal or near normal, although treatable coronary artery disease is not an absolute contraindication. Ventilator support prior to transplantation is a significant risk factor.7 Pulmonary neoplasia is generally regarded as a contraindication because of its early dissemination but bronchioloalveolar carcinoma (now termed adenocarcinoma-in-situ) is often limited to the lungs and double-lung transplantation has been employed to treat this form of lung cancer.8 Contraindications of less importance include age over 65 years and body mass index over 30.
In the USA, priority is given to those patients with rapidly progressive conditions such as idiopathic pulmonary fibrosis and who are in most need of a lung transplant. This is achieved with a numerical scoring system that takes into account the probability and duration of survival without a transplant as compared to that following transplantation. The system, which was introduced in 2005, has resulted in increased numbers of patients with idiopathic pulmonary fibrosis receiving transplants and a decrease in both waiting-list mortality and waiting-list time.9,10 However, a high score predicts increased morbidity and mortality following transplantation, reflecting the poor clinical condition of many of the patients transplanted.11,12 Such a system has yet to be widely adopted: in the UK organs are currently allocated on the basis of proximity and time on the waiting list.
Donor matching
The selection and management of donors and preservation of the harvested lung are crucial to reducing the impact of brainstem death on the lung and minimising reimplantation injury. Brainstem death leads to adrenergic systemic hypertension, which results in fluid shifting to the lesser circulation and neurogenic pulmonary oedema. Such injury is part of a generalised release of cytokines and other inflammatory mediators that damage many organs, including the lungs, where it may compound the other causes of injury, so that only about 20% of donated cadaver lungs can be used.13
Current donor selection criteria include age less than 65 years, ABO compatability, a clear chest X-ray, no airway mucosal inflammation at bronchoscopy and no history of lung contusion, severe chest trauma, previous cardiopulmonary surgery or aspiration.14 Donor seropositivity for cytomegalovirus is dangerous in a seronegative recipient.7 Persistent hepatitis B and C antigenaemia and human immunodeficiency virus infection are further contraindications. However mild asthma and smoking do not adversely affect outcome.15 Physiotherapy, bronchial toilet, prophylactic antibiotics as required and monitoring of blood gases and pulmonary wedge pressure are all important in managing a potential donor. Harvested lungs are best stored inflated, cooled and flushed through the pulmonary artery with Euro-Collins solution. Ischaemia is tolerated for up to 8 hours.14 Donor–recipient matching includes an approximate similarity in chest size, although volume reduction surgery may circumvent this if there is a significant mismatch. One lobe of an adult’s lung may be used to replace a whole lung of a child.16 Tissue transplanted into a child grows at a normal rate.17
Potential recipients are screened for donor-specific lymphocytoxic antibodies against a panel of HLA-A, -B and -DR antigens.17a The result is considered positive if the recipient’s serum reacts to more than 10% of the HLA antigens in the panel, in which case donor-specific T- and B-lymphocytotoxic antibody cross-matching is required. A positive result here contraindicates transplantation from that donor as it is associated with accelerated graft rejection.18
Role of the histopathologist
Histopathology plays a major role in assessing the postoperative complications of transplantation, but can contribute more than this to the management of transplant recipients, both before and after the operation (Box 11.1).19,20 An accurate preoperative diagnosis is important because histopathological reassessment of the potential recipient may affect the choice of transplantation procedure and identify conditions treatable by other means or that need to be eradicated before transplantation is undertaken. It may also predict the presence of systemic disease and the likelihood of the original disease recurring in the allograft, or detect malignant disease, which apart from bronchioloalveolar carcinoma (now adenocarcinoma-in-situ) is an absolute contraindication to transplantation. Explanted lungs should also be examined, and without delay, in case they show unsuspected diseases such as tuberculosis, sarcoidosis or malignancy that may be active elsewhere in the recipient.21 A major discrepancy between the pretransplant diagnosis and that apparent on examining the explant has been reported in up to 10% of cases.22 Similarly, if only one donor lung is used, the other should be examined as this may identify unsuspected infection or other disease that is likely also to be present in the graft. Thus, histopathologists should not be concerned solely in assessing complications, but this is undoubtedly their major role and the rest of this chapter will be largely confined to this aspect of transplantation.
Complications
To assess complications, at least five pieces of alveolated lung should be examined at a minimum of three levels each, using connective tissue and fungal stains in addition to haematoxylin and eosin. Because sequential sampling is common, the possibility of observed abnormalities being due to previous biopsies always has to be considered. It is also important to remember that some patterns of lung injury are non-specific. For example, diffuse alveolar damage may reflect severe reperfusion injury, infection or acute rejection. It may require special stains for infective agents and a consideration of the timing of events to distinguish these causes. There may be both rejection and infection and as their histological features overlap the biopsy findings should always be interpreted in the light of the clinical and radiological features, previous biopsy findings and results of current microbiological and serological investigations. The principal complications are listed in Box 11.2 and will now be considered.
Box 11.2 Complications of lung transplantation
Perioperative allograft injury
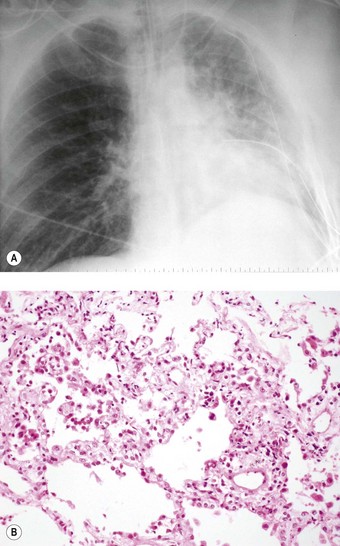
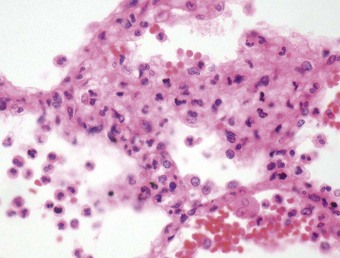
Even if the operation goes well and there are no surgical complications, the early postoperative period is often marked by a temporary period of dyspnoea. Chest radiographs at this time often show hilar opacification and if biopsy is undertaken this generally shows pulmonary oedema accompanied by a mild neutrophil exudate (Fig. 11.2).23 These changes, termed reimplantation injury, are variously attributed to deterioration of the graft, surgical trauma, ischaemia, severance of the pulmonary lymphatics and the release of free radicals and chemokines from neutrophils interacting with the graft endothelium damaged by ischaemia.24 They usually appear within 48 hours and peak at day 4, slowly settling as lymphatics regenerate and lung drainage is re-established.
More severe changes include those of diffuse alveolar damage, to which the term ‘primary graft failure’ has been applied.25 This may represent a reimplantation response, rejection or infection or occur for no obvious cause. The risk factors include those relating to the donor (advanced age, smoking history, prolonged ventilation and duration of ischaemia), the recipient (transplantation for pulmonary hypertension and pulmonary fibrosis) and the operation (cardiopulmonary bypass and excessive use of blood products).26 Primary graft failure may progress to parenchymal fibrosis. It is associated with reduced survival at 1 year.27
Airway infection promoted by slow recovery of mucociliary clearance may also complicate the early postoperative period,28,29 while alveolar proteinosis and an unusual neutrophil-rich pattern of mixed interstitial pneumonitis have also been described.30,31
Complications involving the airway anastomosis
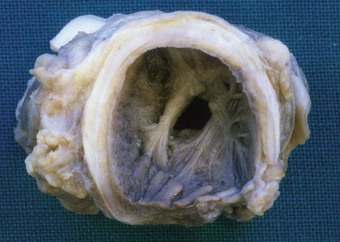
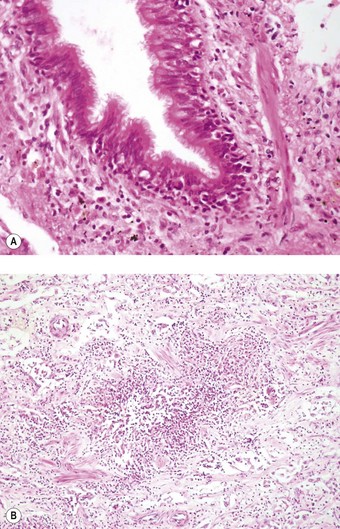
Apart from the combined heart–lung procedure, lung transplantation inevitably involves severance of the tracheobronchial blood supply so that the donor airway receives only retrograde pulmonary arterial blood until new bronchial arterial anastomoses develop. It is not surprising therefore that poor healing at the airway anastomosis has been a serious complication, although bronchial artery revascularisation, performed at a few centres at the time of transplantation, have rendered it less common.32,33 The anastomosis is also subject to infection until the surface epithelium heals and mucociliary clearance recovers.29 Denervation contributes to the risk of infection by eliminating the cough reflex and promoting aspiration.34 Dehiscence of the airway anastomosis is a devastating early complication, but one that is fortunately now rare. More common is the development of exuberant granulation tissue. This may seriously narrow the airway and require endoscopic removal or cryotherapy.35 Late airway complications include stenosis and bronchomalacia (Figs 11.3 and 11.4), requiring the insertion of stents.36
Complications involving the vascular anastomosis
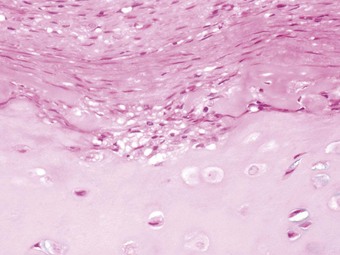
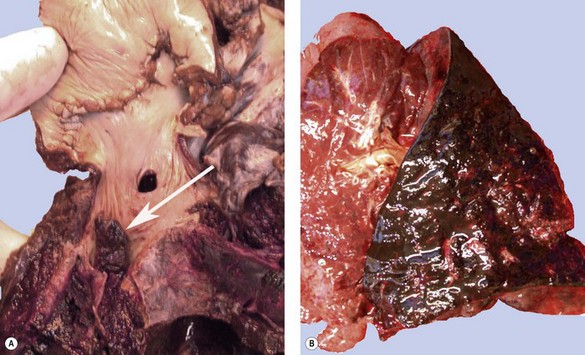
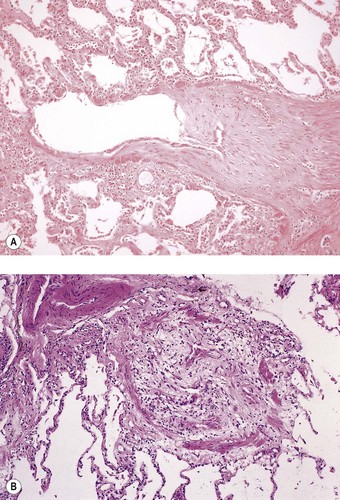
Postoperative obstruction of the pulmonary artery anastomosis is rare but kinking may occur when the vessels are long. This usually develops in the first week. It may be diagnosed by ventilation/perfusion scan or arteriography. Venous obstruction may also develop early after transplantation, especially when size mismatch leads to torsion of a small donor lung on its vascular pedicle. The result may be thrombosis of one or more of the pulmonary veins with infarction of the corresponding lobe (Fig. 11.5).37–39 Systemic embolisation may also occur if the thrombus extends into the left atrium and fragments. Diagnosis is by transoesophageal echocardiography.38,40 Early recognition of this potentially catastrophic complication is essential if early surgical intervention with salvage of the graft (and patient) is to be achieved.
Rejection
The mechanisms underlying lung allograft rejection are not fully understood but the process is probably initiated by T-cell recognition of histocompatibility antigens on the surface of donor cells.41 The brunt of the rejection damage is on the blood vessels and airways, presumably reflecting the increased expression of histocompatibility antigens by the pulmonary vascular endothelium and airway epithelium that has been shown to follow transplantation.41 Incompatibility is followed by the activation and sequestration of platelets, neutrophils and macrophages within the pulmonary capillaries, the release of reactive oxygen species and inflammatory cytokines, and increased expression of endothelin and inducible nitric oxide synthase.42–44 The rejection process probably involves both antibody and cell-mediated immune mechanisms but the extent to which these each contribute varies: hyperacute rejection is generally regarded as being antibody-mediated whereas acute and chronic rejection are thought to be predominantly cell-mediated.
Hyperacute allograft rejection
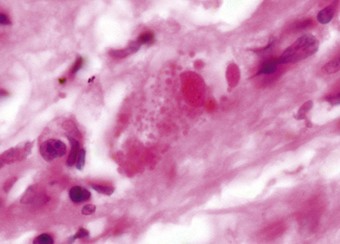
Microscopy shows marked pulmonary congestion and oedema, alveolar haemorrhage, vascular thrombosis, neutrophil infiltration, endothelial and epithelial damage, and ultimately diffuse alveolar damage (Fig. 11.6). The diagnosis is confirmed by the detection by immunofluorescence of C4d complement split product (as a marker of complement activation) and IgG deposition on endothelium, along vessel walls and in alveolar spaces, and by a strongly positive IgG-mediated lymphocytotoxic reaction to donor T and/or B lymphocytes.46,47
Acute allograft rejection
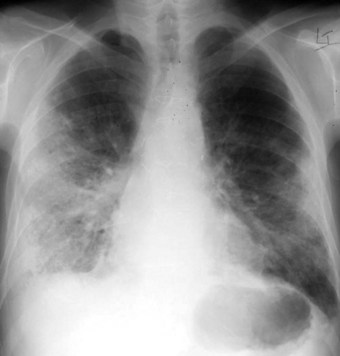
Acute rejection is characterised clinically by dyspnoea, fever, hypoxaemia and pleuropulmonary opacification (Fig. 11.7). It is most frequent in the first postoperative year, typically developing within a few months of the operation but sometimes within a few weeks or several years later.
Acute and chronic rejection are classified and graded according to internationally agreed histological criteria (Box 11.3).48 Acute rejection may be centred on the blood vessels or the airways but predominantly affects the former and the surrounding lung parenchyma. The vascular changes (class A) are treated separately from those involving the airways (class B) because the latter may also reflect chronic infection, ischaemia, aspiration or chronic rejection.28,34,49,50
Box 11.3 Revised working formulation for the classification and grading of pulmonary allograft rejection48
Histologically, the vascular changes are characterised by an infiltrate of variable intensity comprising small lymphocytes, plasma cells, histiocytes and occasional neutrophils surrounding small blood vessels and infiltrating the adjacent alveolar interstitium. They are graded as minimal (grade A1) if they can hardly be discerned without high magnification and mild (grade A2) if they are just evident at low magnification and consist of an infiltrate that includes large activated lymphocytes with pyroninophilic cytoplasm and angulated nuclei. Neutrophils and eosinophils are also seen and there is infiltration of the vascular intima (lymphocytic intimitis or endothelialitis). Endothelial cells show hyperplasia. Moderate acute rejection (grade A3) is characterised by extension of the infiltrate into the alveolar interstitium, while in severe (grade A4) acute rejection, which is usually fatal, the infiltrate is widespread and accompanied by haemorrhagic oedema, increased numbers of alveolar macrophages, fibrinous exudates, hyaline membranes and destructive changes typical of diffuse alveolar damage (Fig. 11.8). Grade A3 and A4 rejection may also be associated with organising pneumonia.49,51–53 With successful suppression of the rejection, follow-up biopsies show a reduction in the infiltrate (termed resolving rejection/lower grade) and a change in its makeup to a mixture of small lymphocytes and haemosiderin-laden macrophages (termed resolved rejection or grade A0).54
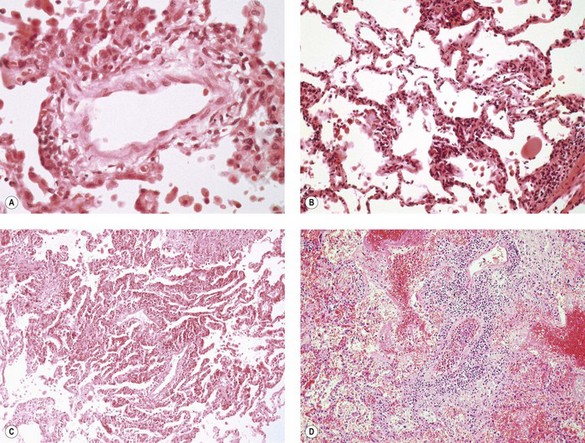
Figure 11.8 Acute parenchymal rejection. (A) Grade A1 (minimal) changes are not discernible at low magnification but high power shows a sparse perivascular lymphoid infiltrate. (B) Grade A2 (mild) shows a more marked infiltrate but this is still restricted to the vessels and the alveolar septa are spared. (C) Grade A3 (moderate) acute rejection showing spread of the infiltrate into surrounding alveolar septa. (D) Grade A4 (severe) acute rejection is characterised by diffuse alveolar damage and a dense perivascular lymphoid infiltrate.
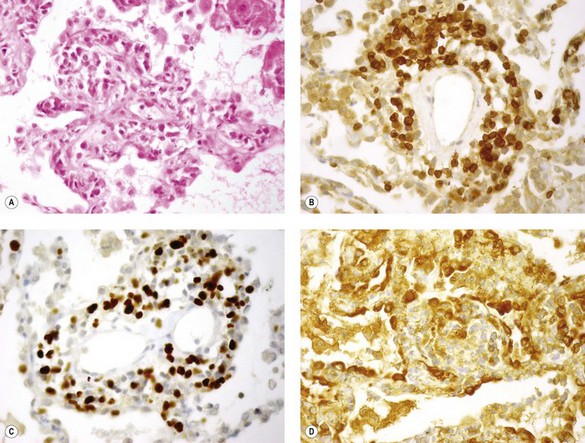
Immunohistochemistry shows that the majority of the lymphoid cells are CD8-suppressor lymphocytes (Fig. 11.9).55 They are often pyroninophilic and may express the lymphocyte activation antigen CD30 and the proliferation antigen Ki-67. B lymphocytes are usually sparse; larger numbers may reflect rejection based on humoral rather than cellular mechanisms and therefore predict a poor response to the usual cell-based immunosuppressive regimes.56 However, their presence should also prompt consideration of other entities such as eosinophilic pneumonia, fungal infection and lymphoproliferative disease.57,58 The vascular endothelium and alveolar epithelium both show upregulation of class II HLA (HLA-DR) antigens.41
Although perivascular lymphoid cell infiltration is the hallmark of acute rejection, similar infiltrates may be seen in patients with infections and other conditions for which augmented immunosuppression is inappropriate. For this reason acute rejection should only be diagnosed and graded in the absence of infection.48 The opportunistic infections may be viral, particularly herpes simplex and cytomegalovirus, the distinctive inclusions of which may be modified by prior antiviral treatment and therefore difficult to identify (Fig. 11.10).59 However, the infiltrates of a viral infection are generally more extensive and the pattern is predominantly that of an interstitial pneumonitis with secondary involvement of vessels.59 Perivascular lymphoid infiltrates may also be seen in Pneumocystis jirovecii pneumonia.60 and in other fungal and bacterial infections, necessitating special stains, and in difficult cases immunocytochemistry and in situ hybridisation.61,62 Eosinophilic pneumonia is an uncommon manifestation of graft rejection and, before accepting it as such, infection by organisms such as Aspergillus should be excluded.63,64 A predominantly neutrophilic infiltrate, necrosis and granuloma formation all favour infection rather than rejection.
Acute rejection should also be distinguished from the ischaemia–reperfusion injury described above and from the lymphoproliferative disorders described below.58 Ischaemia–reperfusion injury lacks the perivascular and interstitial infiltration of rejection while a polymorphous, perivascular infiltrate of B and T lymphocytes with a predominance of the latter favours rejection rather than lymphoproliferative disease, which is characterised by a monomorphous infiltrate of large B lymphocytes.57 Mass lesions also favour lymphoproliferative disease or infection rather than rejection. Recurrence in the allograft of diseases such as sarcoidosis and Langerhans cell histiocytosis may also be misinterpreted as acute rejection if their characteristic features are not well represented.
Airway inflammation in the form of lymphocytic bronchiolitis is the other major pattern of acute rejection. Evidence that it represents rejection includes its progression to chronic rejection (bronchiolitis obliterans) and frequent response to augmented immunosuppression.65 It may accompany or succeed the perivascular infiltrates described above or be seen alone, but it most frequently follows the vascular changes.28,50 Airway inflammation may be graded in the same way as the perivascular infiltration but in practice the small size of the biopsies generally precludes the precision required for this. The changes range from sparse airway cuffing by small lymphoid cells (grade B1R) to diffuse infiltration of the lamina propria and epithelium by medium and large lymphoid cells and epithelial apoptosis (grade B2R) (Fig. 11.11). In severe cases the lymphoid infiltrate is particularly dense and there is ulceration and fibrinopurulent exudation. The importance of large-airway inflammation in rejection is unclear as it is more often secondary to infection. Grading of airway inflammation is therefore currently restricted to the bronchioles.
The differential diagnosis of airway inflammation includes the presence of bronchus-associated lymphoid tissue of donor origin, low-grade infection and the consequences of aspiration. Bronchus-associated lymphoid tissue is distinguished by it comprising aggregates of B lymphocytes, sometimes with admixed anthracotic macrophages, in contrast to the T-cell-rich infiltrates of rejection. Prominent neutrophil or eosinophil infiltration is suggestive of infection or aspiration. However, it is important to note that airway or parenchymal infection, notably by cytomegalovirus, may accompany rejection.66
Chronic allograft rejection
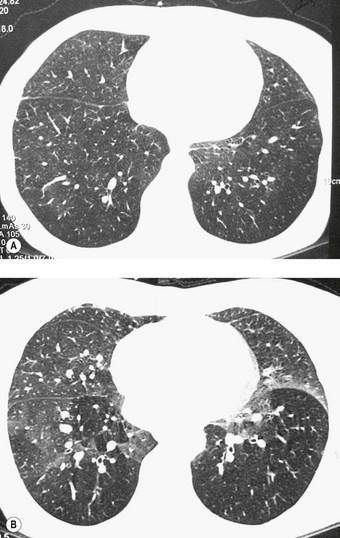
Chronic rejection of the transplanted lung is marked by progressive breathlessness, cough, which is often productive, fever and a decline in lung function.67 The changes, which can be monitored by spirometry and imaging (Fig. 11.12), are centred on the bronchioles (class C) and blood vessels (class D).
The histological features are those of constrictive bronchiolitis obliterans. Typically there is concentric or eccentric hyaline fibrous thickening of the bronchiolar submucosa, encroaching on the airway lumen and eventually resulting in total occlusion (Fig. 11.13).68–71 The changes may be subtle, in which case an elastin stain can be invaluable in identifying the fibrosed bronchioles. Residual bronchiolar epithelium may show squamous metaplasia and there is usually disruption of the muscle coat. The process may be active (i.e. associated with lymphocytic bronchiolitis) or inactive (consisting of dense fibrous scarring with minimal or no inflammation), although this is of no prognostic significance and does not predict response to treatment. The infiltrating cells are T lymphocytes.72 The process is patchy and therefore not always evident in small biopsies,73,74 but its presence may be suggested by obstructive features such as the accumulation of foamy macrophages in distal air spaces. In the current classification constrictive bronchiolitis is simply recorded as being present (C1) or absent (C0).
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree