Chapter 9 Pulmonary eosinophilia
Pulmonary eosinophilia is a clinical term used to describe the association of radiographic lung opacities and blood eosinophilia.1 Synonyms include ‘pulmonary infiltrates with eosinophilia’ and ‘PIE syndrome’.2 Some lung diseases that are characterised by an eosinophilic lung infiltrate are not associated with blood eosinophilia and are therefore dealt with elsewhere – eosinophilic granuloma (now termed Langerhans cell histiocytosis) on page 288 and reactive eosinophilic pleurisy on page 711. Other eosinophilic infiltrates are associated with blood eosinophilia but not with radiographic opacities, e.g. asthma. The eosinophilia–myalgia syndrome seen with the ingestion of certain tryptophan preparations may well have been considered in this chapter but is dealt with under drug reactions (see p. 389).
Aetiology
Aetiologically, pulmonary eosinophilia may be classified as cryptogenic or of known cause, the latter including allergy to fungi, parasites or drugs (Box 9.1).
Allergic aspergillosis is a common cause of pulmonary eosinophilia in the UK whereas infestation by metazoan parasites is commoner in communities in which these parasites are prevalent (Box 9.2). In some parts of the world filariasis is the cause: this is tropical pulmonary eosinophilia, which can be readily diagnosed from the patient’s history of residence in the tropics, by the presence of extraordinarily high levels of both serum IgE and antifilarial antibodies, and by the dramatic therapeutic response to filaricides.3–5 Pulmonary eosinophilia is also caused by a number of drugs, notably nitrofurantoin but occasionally sulphonamides and aspirin, amongst others (Box 9.3). Pulmonary eosinophilia may also be a manifestation of rheumatoid disaease.6
Box 9.3 Drugs causing eosinophilic lung disease
Leukotriene antagonists are not included as it is suspected that their association with Churg–Strauss syndrome represents an unmasking of this condition when these drugs are substituted for corticosteroids.73 GM-CSF, granulocyte–macrophage colony-stimulating factor.
In other patients the cause of the pulmonary eosinophilia is not recognised: such cases are categorised as suffering from cryptogenic pulmonary eosinophilia.7 A majority of these patients are atopic, giving a history of rhinitis or asthma.8 There is a high level of activated helper T lymphocytes in their bronchoalveolar lavage fluid.9 They also show a transient increase in circulating immune complexes.10 The Churg–Strauss syndrome of allergic angiitis and granulomatosis may be regarded as a subset of cryptogenic pulmonary eosinophilia but the hypereosinophilic syndrome is quite distinct from all these reactive forms of eosinophilia and probably represents a leukoproliferative disorder.
Pathogenesis
The eosinophil leukocyte is central to the pathogenesis of all the diseases in this chapter. Eosinophil leukocytes are produced in the bone marrow under the control of cytokines secreted by type 2 helper lymphocytes, notably interleukin-5. Once released into the blood they normally remain there for 12–18 hours, maintaining a count of 50–250/µl. An absolute count is more meaningful than the percentage in a differential white blood cell count. They enter the tissues under the influence of chemotactic agents known as eotaxins,11 of which three are now recognised. These agents are small proteins produced by a variety of cells, which in the lung include bronchial epithelial cells, smooth-muscle cells and fibroblasts, acting under the influence of interleukins derived from type 2 helper lymphocytes and mast cells (see Fig. 3.30, p. 115).11–13
Tissue eosinophilia does not necessarily correlate with blood eosinophilia even though the two usually go together: blood counts may dip as eosinophils flood the lung and then rise as the infiltrate resolves due to a lag in bone marrow response. Sputum and bronchoalveolar lavage counts reflect lung tissue eosinophilia better than blood counts but, like neutrophils, eosinophils appear to be washed out of the lung more easily than macrophages so that raised lavage counts are encountered in diseases such as idiopathic pulmonary fibrosis that are seldom characterised by any appreciable degree of tissue eosinophilia. Bronchoalveolar lavage eosinophils are usually expressed as a percentage of total cells recovered from the lungs, the normal being less than 3%. Eosinophils in sputum are generally expressed only in broad qualitative terms (e.g. ‘small, moderate or large numbers’). When there is eosinophilic pneumonia, eosinophils are the predominant cell in both sputum and bronchoalveolar lavage fluid. They are represented at levels greatly in excess of those found in diseases such as idiopathic pulmonary fibrosis.
Once in the tissues, eosinophils secrete a number of products, some of which counter the action of substances secreted by mast cells. Histaminase and aryl sulphatase, for example, respectively destroy histamine and the leukotriene complex formerly known as slow-reacting substance of anaphylaxis. Eosinophils also phagocytose immune complexes and mast cell granules, and tend to dampen reaginic hypersensitivity reactions. However, they also secrete substances such as reactive oxygen radicals, major basic protein and eosinophil cationic protein which, whilst helping to eliminate parasites, are also toxic to host cells (Fig. 9.1).14–17 These eosinophil products are thought to be responsible for much of the tissue damage in the eosinophilic disorders about to be described. Activation of the cells involved in cryptogenic eosinophilic pneumonia is suggested by the demonstration that they express intercellular adhesion molecules, probably under the influence of the activated T lymphocytes referred to above.18 Electron microscopy shows that released eosinophil granules are closely associated with degenerate and necrotic alveolar tissue.19
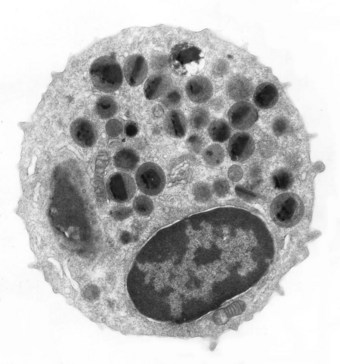
Figure 9.1 Electron micrograph of an eosinophil. A bilobed nucleus is surrounded by granules, many of which have a bar-shaped electron-dense core. The granule core consists of a basic protein whilst the surrounding matrix contains cationic protein, both of which are highly toxic.14
(Courtesy of Miss A Dewar, Brompton, UK.)
Corticosteroids act on eosinophils in a variety of ways.20 They cause rapid eosinophil sequestration in peripheral blood vessels and inhibit their production in the bone marrow. They also reduce eosinophil survival by blocking lymphokines that inhibit eosinophil apoptosis. Eosinophil adherence and chemotaxis are also diminished by corticosteroids. In view of these many actions it is not surprising that corticosteroids are effective in treating many diseases characterised by eosinophilia.
Simple pulmonary eosinophilia
The Swiss physician Löffler described two conditions that were characterised by blood eosinophilia. One is the hypereosinophilic syndrome, which is dealt with below. The other is one that later came to be known as simple pulmonary eosinophilia.21 Rarely a patient may display features of both conditions.22 The term ‘Löffler’s syndrome’ is confusingly applied to both and therefore best avoided.
Chronic eosinophilic pneumonia
Chronic eosinophilic pneumonia is a serious condition which generally requires treatment with corticosteroids.8,20,23–26 These usually prove efficacious but may have to be continued for many months, or even indefinitely, to prevent relapse. The disease is occasionally life-threatening. The peak incidence is in the fifth decade and females preponderate 2:1. There is often a history of atopic illnesses such as rhinitis or asthma and the condition is regarded as having an allergic basis. Any of the aetiological agents outlined above may be responsible, drugs, fungi or parasites, or the cause may not be identified.
Clinical features
The onset is insidious, with gradually worsening cough, dyspnoea, fever and weight loss. There is blood and sputum eosinophilia. However, blood and tissue eosinophil levels are not always elevated contemporaneously, the raised blood counts often having abated whilst eosinophils are still evident in the lung tissue. Bronchoalveolar lavage cell counts correlate better with the tissue findings, eosinophils being the predominant cell in lavage fluid from an involved segment.13 The chest radiograph is distinctive, showing bilateral opacification that is peripheral and apical.27 It has been likened to the plume of a volcano or the photographic negative of the perihilar shadowing of pulmonary oedema. This is mirrored by subpleural consolidation on high-resolution computed tomography.
Pathology
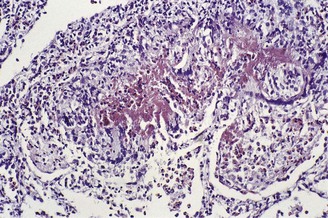
In the occasional case that comes to autopsy the lungs are found to be heavy and firm with irregular areas of pale consolidation (Fig. 9.2). Microscopically, eosinophils fill the alveoli and infiltrate the interstitium (Fig. 9.3).8,23,28 The alveoli also contain a variable number of macrophages, some of which may be multinucleate (Fig. 9.4). The macrophages may outnumber the eosinophils to mimic the appearances of desquamative interstitial pneumonia (Fig. 9.5). Focal collections of eosinophils may undergo necrosis to form so-called eosinophil abscesses, which are sometimes attended by foreign-body-type giant cells engulfing eosinophilic debris (Fig. 9.6).8 Sparse sarcoid-like granulomas are occasionally seen, and in rare cases are unduly prominent.29 The eosinophil infiltrate may involve small blood vessels but necrotising vasculitis is not encountered: its presence would suggest Churg–Strauss allergic granulomatosis, which is dealt with below. Knowledge of treatment is important as eosinophils diminish with corticosteroid administration. Healing, whether occurring spontaneously or in response to such treatment, usually results in complete resolution but may be by repair, this resulting in alveolar fibrosis that is indistinguishable from any other organising pneumonia.10,30
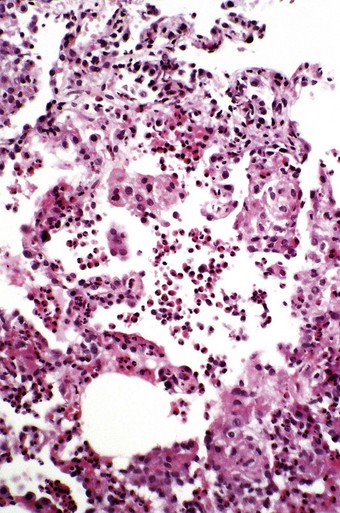
Figure 9.3 Eosinophilic pneumonia. The alveoli contain many eosinophil polymorphonuclear leukocytes.
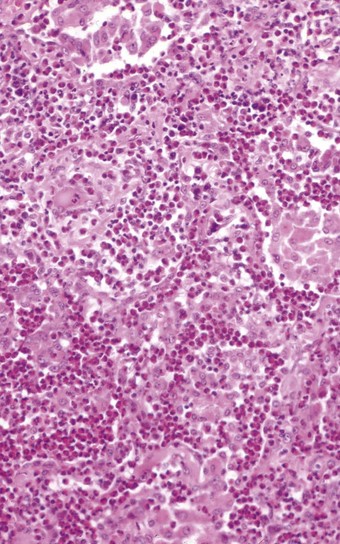
Figure 9.4 Eosinophilic pneumonia in which the alveoli are filled by both eosinophils and macrophages.
(Courtesy of Dr T Jelihovsky, Sydney, Australia.)
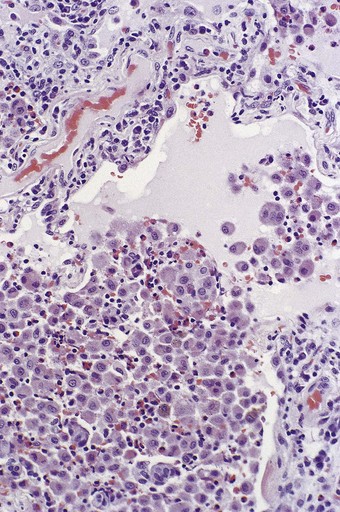
Figure 9.5 Eosinophilic pneumonia. There is moderate eosinophil exudation but these cells are outnumbered by alveolar macrophages.
Differential diagnosis
The location of the eosinophils in air spaces as well as in the interstitium helps to distinguish eosinophilic pneumonia from eosinophilic granuloma (Langerhans cell histiocytosis), as do the presence of eosinophils in the blood, sputum and bronchoalveolar lavage fluid and a lack of Langerhans cells aggregation. Pneumothorax may provoke a reactive eosinophilic infiltrate but this is limited to the alveoli immediately beneath the pleura.
Acute eosinophilic pneumonia
Clinical features
The term ‘acute eosinophilic pneumonia’ was introduced in 1989 for a condition characterised by the sudden onset of a febrile illness accompanied by myalagia, pleuritic pain and hypoxaemia.31,32 Patients may be of any age and either sex.33 Chest radiographs show extensive bilateral opacification and effusions.27 Blood eosinophil counts are often normal but many eosinophils are found on bronchoalveolar lavage and pleural aspirates. A history of asthma is unusual but there may be one of allergic rhinitis.34 Apart from the lavage and aspirate findings, the clinical features suggest severe infection or the acute respiratory distress syndrome. The condition is life-threatening but responds well to corticosteroids.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree