Transhiatal Esophagectomy Without Thoracotomy
Mark B. Orringer
Introduction
In the mid-1970s, the technique of transhiatal esophagectomy (THE) without thoracotomy and cervical esophagogastric anastomosis (CEGA) for both benign and malignant disease was rediscovered and popularized. Until that time, a transthoracic esophagectomy with an intrathoracic esophagogastric anastomosis was the standard approach to esophageal resection and reconstruction. With this latter approach, postoperative respiratory insufficiency associated with a combined thoracic and abdominal operation and mediastinitis resulting from an intrathoracic anastomotic leak were the leading causes of operative mortality. The notion that preservation of an intra-abdominal gastric reservoir was important in patients with benign esophageal disease was also prevalent. The purported advantages of the THE were (a) avoidance of a thoracotomy (and therefore less postoperative respiratory distress) and (b) a CEGA (in which an anastomotic leak resulted in a salivary fistula, which was well managed by open drainage and was seldom associated with mediastinitis and its 50% mortality). THE was initially criticized because of concern about inadequate hemostasis in mobilizing the esophagus from the mediastinum “blindly” and failure to permit an en bloc lymph node dissection felt important in other oncologic operations.
Over the past 30 years, the technique of THE has been progressively refined, and the author now regards this approach as the procedure of choice in the majority of patients requiring esophageal resection and reconstruction for both benign and malignant esophageal disease. The technical refinements in mobilizing and handling the stomach, dissecting the esophagus transhiatally, preparing the gastric conduit, positioning the stomach within the posterior mediastinum, and constructing the CEGA are reviewed in this chapter.
Pathophysiology
Most often, patients requiring esophageal resection and reconstruction suffer from esophageal obstruction, which not only has nutritional consequences from impaired caloric intake but is also associated with pulmonary sepsis associated with impaired swallowing. Hypovolemia and dehydration are common. People produce 1 to 1.5 L of saliva each day, and the patient who is unable to swallow saliva comfortably and is constantly regurgitating and expectorating saliva that will not pass freely through the esophagus may become severely hypokalemic.
THE is indicated in most conditions for which esophageal resection and reconstruction is required. In 2007, the author and his associates reported a series of 2,007 transhiatal esophagectomies, 1,525 of which (76%) had been performed for carcinoma of the intrathoracic esophagus and 482 (24%) for a wide variety of benign conditions (Table 1). Although an esophagomyotomy is a highly effective procedure for the treatment of achalasia, this neuromotor esophageal abnormality is not curable, and patients seen after a failed esophagomyotomy, a reflux stricture that has developed after an esophagomyotomy without an antireflux procedure, or those with a megaesophagus of advanced achalasia are
candidates for esophagectomy. With the advent of histamine-2 blockers and proton pump inhibitors, the number of esophageal reflux strictures requiring resection has dramatically fallen. On the other hand, the dramatic increase in the number of laparoscopic fundoplications performed for control of gastroesophageal reflux and repair of hiatal hernias and recurrent hiatal hernias has generated an increasing number of failed fundoplications that are becoming a more common indication for esophagectomy. In patients with severe dysphagia or refractory esophagitis after two failed hiatal hernia repairs, the likelihood of achieving lasting reflux control and relief of dysphagia with a third antireflux operation is so low that the author advises an esophagectomy at this point. Clinically significant gastroesophageal reflux following a properly performed CEGA is uncommon, and with no esophagus, the patient is no longer symptomatic from esophagitis. The decision to perform an esophagectomy for recurrent reflux or hiatal hernia, however, is a difficult one, and patients must understand that the outcome may include cervical dysphagia, reflux from the intrathoracic stomach, and dumping symptoms.
candidates for esophagectomy. With the advent of histamine-2 blockers and proton pump inhibitors, the number of esophageal reflux strictures requiring resection has dramatically fallen. On the other hand, the dramatic increase in the number of laparoscopic fundoplications performed for control of gastroesophageal reflux and repair of hiatal hernias and recurrent hiatal hernias has generated an increasing number of failed fundoplications that are becoming a more common indication for esophagectomy. In patients with severe dysphagia or refractory esophagitis after two failed hiatal hernia repairs, the likelihood of achieving lasting reflux control and relief of dysphagia with a third antireflux operation is so low that the author advises an esophagectomy at this point. Clinically significant gastroesophageal reflux following a properly performed CEGA is uncommon, and with no esophagus, the patient is no longer symptomatic from esophagitis. The decision to perform an esophagectomy for recurrent reflux or hiatal hernia, however, is a difficult one, and patients must understand that the outcome may include cervical dysphagia, reflux from the intrathoracic stomach, and dumping symptoms.
Table 1 Indications for Transhiatal Esophagectomy (2,007 Patients) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
In the author’s experience with more than 2,000 transhiatalesophagectomies, this approach has been applicable in 98% of all patients requiring esophageal resection. However, clear contraindications to THE, or utilizing the stomach as an esophageal replacement exist. Patients with upper or middle third esophageal carcinoma, who have tracheobronchial invasion documented by bronchoscopy and biopsy, magnetic resonance imaging, computed tomography, or esophageal endoscopic ultrasonography, are not candidates for a THE. Similarly, the author will not proceed with a THE in any patient with documented distant (M1) metastatic esophageal cancer. If the patient has undergone a prior esophageal operation (fundoplication, esophagomyotomy, perforation repair), safe transhiatal mobilization of the esophagus may prove to be impossible, and the surgeon must be prepared to convert to an open thoracotomy and transthoracic esophagectomy. When carcinoma of the cardia and upper stomach may necessitate resection of a major portion of the stomach to achieve an adequate margin, the suitability of the colon as an esophageal substitute should be determined with a preoperative barium enema examination and a colon bowel prep carried out in the event that a colonic interposition is required. Without question, the most important contraindication to proceeding with a THE is the surgeon’s assessment at the time of transhiatal mediastinal exploration that the esophagus is so adherent to adjacent vital structures that a transthoracic mobilization of the esophagus under direct vision is safer.
The importance of careful nutritional assessment, medical evaluation, and documentation of adequate pulmonary function and cardiac reserve in a successful outcome after THE cannot be overstated. Aggressive preoperative preparation of the patient for THE is consistently rewarded by decreased morbidity and mortality. The author is adamant that patients completely abstain from cigarette smoking for a minimum of 3 weeks before operation, even in those with carcinoma. An incentive inspirometer is issued to the patient at the time of the first consultation, and regular daily pulmonary physiotherapy is instituted before the patient is admitted for esophagectomy. Patients are instructed to walk 1 to 3 miles daily prior to their scheduled operation, and the importance of early postoperative ambulation is emphasized. If esophageal obstruction is severe, a nasogastric feeding tube is inserted, and administration of supplemental tube feedings at home is initiated. Patients are typically admitted for their esophagectomy on the day of the scheduled operation. Preoperative intravenous hyperalimentation is virtually never used, as is the case with hospitalization immediately prior to surgery.
THE is performed in four phases: abdominal, cervical, mediastinal, and the anastomosis. Typically, after induction of general anesthesia, flexible esophagoscopy is performed to verify the distance of the esophageal pathology from the upper incisor teeth and to document that there is a sufficient length of normal proximal esophagus to
allow construction of a CEGA. After the esophagoscopy, a nasogastric tube is inserted to decompress the stomach and aid in identifying the esophagus during its mobilization in the neck. The head is turned to the right and is supported on a soft head ring, and the neck is extended by placing a small rolled sheet beneath the scapulae. The skin of the anterior neck, chest, and abdomen is prepped and draped from the mandibles to the pubis and anterior to both midaxillary lines. Two large-bore peripheral intravenous lines and a radial artery catheter for intraoperative monitoring of blood pressure are placed and carefully padded as the arms are placed at the patient’s sides. The author prefers to avoid “bumping up one side” to allow performance of an anterolateral thoracotomy if needed. If it is necessary to convert to a transthoracic esophagectomy, this is best performed through a true posterolateral thoracotomy, and the author prefers to temporarily close the abdominal wound, cover it with a plastic surgical drape, and turn the patient to his or her side for performance of the transthoracic mobilization. The chest is then closed, and the patient rolled supine once again for completion of the esophageal reconstruction. A table-mounted self-retaining (upper-hand) retractor optimizes exposure of the upper abdomen for a THE.
allow construction of a CEGA. After the esophagoscopy, a nasogastric tube is inserted to decompress the stomach and aid in identifying the esophagus during its mobilization in the neck. The head is turned to the right and is supported on a soft head ring, and the neck is extended by placing a small rolled sheet beneath the scapulae. The skin of the anterior neck, chest, and abdomen is prepped and draped from the mandibles to the pubis and anterior to both midaxillary lines. Two large-bore peripheral intravenous lines and a radial artery catheter for intraoperative monitoring of blood pressure are placed and carefully padded as the arms are placed at the patient’s sides. The author prefers to avoid “bumping up one side” to allow performance of an anterolateral thoracotomy if needed. If it is necessary to convert to a transthoracic esophagectomy, this is best performed through a true posterolateral thoracotomy, and the author prefers to temporarily close the abdominal wound, cover it with a plastic surgical drape, and turn the patient to his or her side for performance of the transthoracic mobilization. The chest is then closed, and the patient rolled supine once again for completion of the esophageal reconstruction. A table-mounted self-retaining (upper-hand) retractor optimizes exposure of the upper abdomen for a THE.
Abdominal Phase
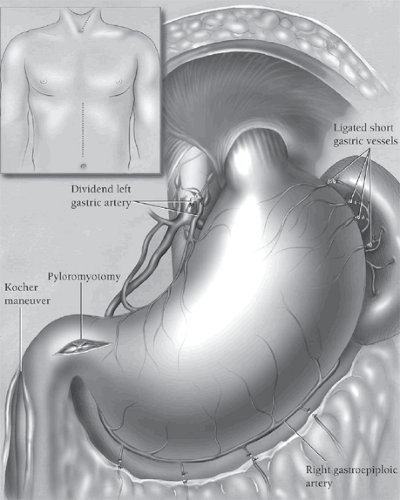
The peritoneal cavity is entered through an upper midline supraumbilical incision (Fig. 1, inset). The triangular ligament of the liver is divided with electrocautery, and the left lobe of the liver is folded downward and retracted to the right with the liver blade of the upper hand retractor. The left abdominal wall is retracted laterally with the standard abdominal wall retractor blade. The stomach is assessed immediately for its suitability as an esophageal substitute. If a prior gastrostomy has been performed, this is taken down from the abdominal wall and the gastrostomy site temporarily closed with a figure-eight chromic suture. The greater omentum along the high greater curvature of the stomach is retracted to the left, and a “clear space” between the stomach wall and the omentum is developed. Once the lesser omental space has been entered behind the greater omentum, fingers inserted into this opening and behind the omentum can be used to define the high short gastric and left gastroepiploic vessels entering the stomach in this region. Several of these vessels are progressively defined, clamped with 13-in.-long right-angled clamps, divided, and secured with 2-0 silk ties. Care should be taken to divide these vessels well away from the gastric wall to avoid ischemic injury, and traction on the stomach should be minimized to avoid trauma to the stomach.
Rather than dividing all of the high short gastric vessels at this point, the dissection is carried inferiorly along the greater curvature of the stomach toward the pylorus, dividing each major left gastroepiploic vessel approximately 1.5 to 2 cm away from the greater curvature of the stomach to minimize trauma to the gastric wall. The termination of the right gastroepiploic artery at its communication with the left gastroepiploic artery is identified. From this point inferiorly along the stomach, care is taken to divide the greater omentum 1.5 to 2 cm inferiorly to the right gastroepiploic artery to preserve patency of this vessel, which is the main blood supply of the mobilized stomach when it is used as an esophageal replacement. Once the greater omentum has been separated from the stomach to the level of the pylorus, the remaining attached omentum along the high greater curvature of the stomach is retracted to the left, and the highest short gastric vessels are mobilized, clamped, divided, and ligated. Care is taken to avoid injury to the spleen; gastric necrosis, which results from ligating the gastroepiploic vessels too near the stomach wall; and contusion of the high greater curvature from excessive traction and retraction of the stomach. When the entire greater curvature of the stomach is freed from its adjacent omentum, adhesions between the stomach and the retroperitoneum are carefully divided with electrocautery to ensure complete gastric mobility.
The peritoneum overlying the esophageal hiatus is incised, and the esophagogastric junction is encircled with a 1-in. rubber drain. Attention is then turned to the lesser curvature of the stomach. One hand is placed behind the stomach from the greater curvature side until the fingers can be seen
through the filmy gastrohepatic omentum along the lesser curvature of the stomach. The gastrohepatic omentum is incised and divided superiorly to the level of the diaphragmatic hiatus with electrocautery. As the gastrohepatic omentum is being divided, it is carefully palpated for an aberrant left hepatic artery arising from the left gastric artery. If the vessel is present, it is preserved. Retraction of the midstomach to the left tenses the soft tissues along the high lesser curvature. The left gastric vein is identified, mobilized, clamped between long right-angled clamps, divided, and ligated with 2-0 silk ties. The adjacent left gastric artery is identified at its origin from the celiac axis. The vessel is doubly ligated toward the celiac artery, clamped, and divided reflecting adjacent lymph nodes to the left with the stomach. The remaining soft tissue adjacent to the lesser curvature of the stomach is progressively divided with electrocautery moving toward the diaphragmatic hiatus so that the soft tissue margin is maximized. With mobilization of the high lesser curvature and entire greater curvature of the stomach now completed, attention is redirected to the diaphragmatic hiatus.
through the filmy gastrohepatic omentum along the lesser curvature of the stomach. The gastrohepatic omentum is incised and divided superiorly to the level of the diaphragmatic hiatus with electrocautery. As the gastrohepatic omentum is being divided, it is carefully palpated for an aberrant left hepatic artery arising from the left gastric artery. If the vessel is present, it is preserved. Retraction of the midstomach to the left tenses the soft tissues along the high lesser curvature. The left gastric vein is identified, mobilized, clamped between long right-angled clamps, divided, and ligated with 2-0 silk ties. The adjacent left gastric artery is identified at its origin from the celiac axis. The vessel is doubly ligated toward the celiac artery, clamped, and divided reflecting adjacent lymph nodes to the left with the stomach. The remaining soft tissue adjacent to the lesser curvature of the stomach is progressively divided with electrocautery moving toward the diaphragmatic hiatus so that the soft tissue margin is maximized. With mobilization of the high lesser curvature and entire greater curvature of the stomach now completed, attention is redirected to the diaphragmatic hiatus.
The phrenoesophageal attachments and adjacent soft tissue are mobilized away from the diaphragmatic hiatus and toward the distal esophagus and esophagogastric junction with electrocautery, and the lower mediastinum is entered. It may be necessary to resect an adjacent rim of the diaphragmatic hiatus if tumor is adherent to the muscle at this point. Insertion of a narrow “heart” retractor into the hiatus anteriorly and upward traction on it exposes the distal esophagus within the posterior mediastinum and allows mobilization of the soft tissue on either side toward the esophagus. This dissection is typically carried out well into the lower mediastinum using a combination of alternating lateral retraction on the esophagus to tense the contralateral soft tissue, elevation of the tissue with a long right-angled clamp, and division with a needle-tipped electrocautery. As the esophagus is being progressively mobilized from the low mediastinum, attention should be paid to the pleura on either side, as entry into either pleural cavity warrants placement of a chest tube. During its mobilization, the esophagus is grasped and “rocked” from side to side to verify that it is not excessively fixed to adjacent spine, prevertebral fascia, or aorta. In this fashion, mobilization of the distal esophagus and its adjacent periesophageal soft tissue is achieved to the level of the carina. As the dissection is performed, the radial artery blood pressure should be watched carefully to avoid prolonged hypotension resulting from cardiac displacement. Once the distal 10 cm of esophagus has been completely freed from the mediastinum, an abdominal pack is inserted through the diaphragmatic hiatus into the low mediastinum to facilitate hemostasis.
A generous Kocher maneuver is performed until there is sufficient mobilization to allow the pylorus to be elevated from its usual location in the right upper quadrant to a point aligned with the xiphoid process. Two pyloric traction sutures of 3-0 silk are then placed, one on the superior and one on the inferior aspect of the pylorus, and are used to elevate the pylorus into the field in preparation for a pyloromyotomy. Beginning 1.5 to 2 cm on the gastric side of the pylorus, the gastric muscle is elevated from the underlying submucosa with a fine-tipped right-angled clamp and divided with a needle-tipped electrocautery. The muscle incision is carried directly across the pylorus and 0.5 to 1 cm onto the duodenum, exposing the characteristic fine venous plexus that characterizes duodenal submucosa. Inadvertent entry into the pyloroduodenal mucosa is managed by closing the hole with several interrupted 5-0 polypropylene sutures. The pyloromyotomy is not converted to a pyloroplasty in order to avoid a suture line at right angles to the vertical axis of the stomach, which will be pulled upward toward the neck. Hemostatic metallic clips are placed on both traction sutures near the pylorus prior to cutting them in order to identify the level of the pylorus in future radiographic follow-up.
A 14 French rubber jejunostomy feeding tube is inserted 10 to 15 cm distal to the ligament of Treitz and secured in place with a Weitzel maneuver. The jejunostomy tube is not yet brought through the abdominal wall but rather emerges from the inferior end of the abdominal incision and is clamped with a hemostat and covered with a towel, which is clamped on either side to the drapes to minimize the chance of dislodging the feeding tube during the subsequent dissection. With gastric mobilization, dissection of the distal esophagus from the inferior mediastinum, the Kocher maneuver, pyloromyotomy, and feeding jejunostomy completed (Fig. 1), the abdominal phase of the operation ends, and attention is turned to the neck.
Cervical Phase
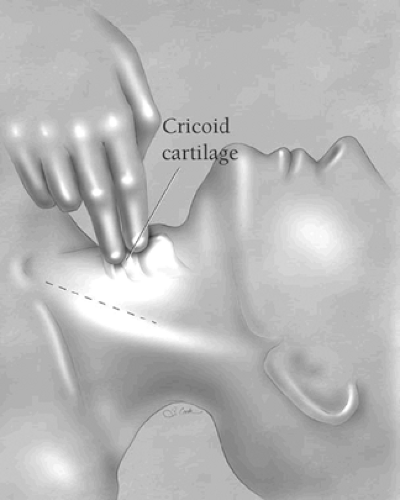
During the cervical phase of the operation, it is preferable that the surgeon stand on the left side of the operating table. The cricoid cartilage is palpated to identify the level of the cricopharyngeus sphincter. A 5- to 7-cm oblique incision paralleling the anterior border of the left sternocleidomastoid muscle is made and extended no more than 2 to 3 cm superior to the cricoid cartilage (Fig. 2). The incision is carried through
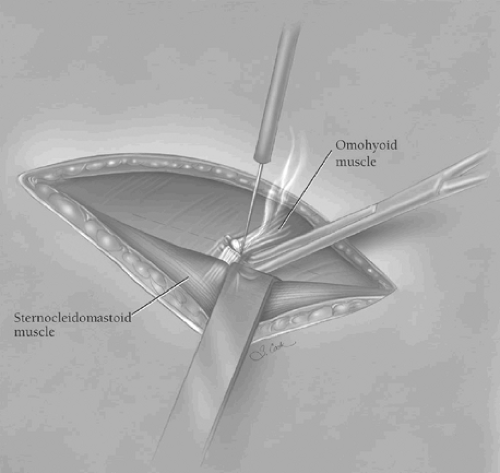
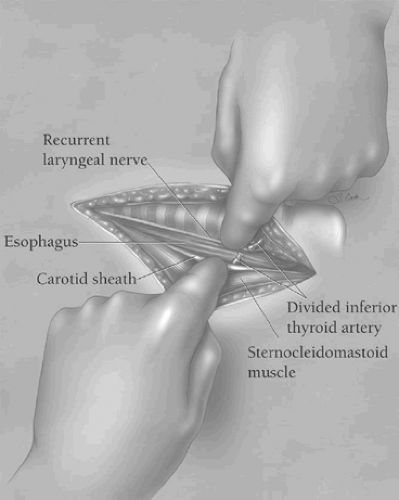
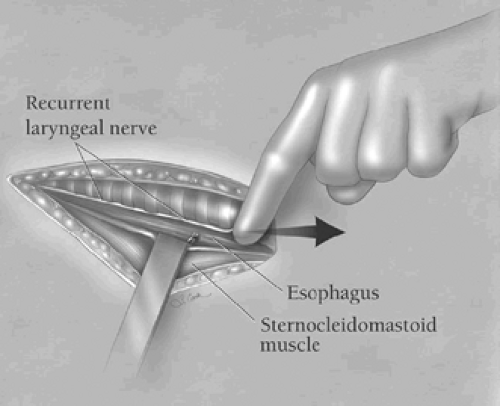
the underlying platysma muscle, and the fascia along the anterior edge of the sternocleidomastoid muscle is incised in the direction of the wound. Blunt finger retraction is used to separate the sternocleidomastoid muscle from the underlying cervical fascia medial to it. Retraction of the sternocleidomastoid muscle laterally allows identification of the underlying omohyoid muscle, the central tendon of which is elevated with a right-angled clamp and divided (Fig. 3). Elevation of the medial belly of the divided omohyoid muscle tenses the contiguous omohyoid fascia layer, which is incised in the direction of the wound superiorly and inferiorly. The omohyoid fascia layer leads to the carotid sheath and its contents, which are gently retracted laterally. The larynx and the trachea are simultaneously retracted medially by the assistant’s index finger (Fig. 4). No metal retractor is placed against the tracheoesophageal groove during any portion of the operation to minimize the risk of recurrent laryngeal nerve injury. As the interior jugular vein is retracted laterally, the middle thyroid vein is often identified and should be mobilized, clamped at either end with fine right-angled clamps, divided, and ligated. Using the cricoid cartilage as a landmark, the inferior thyroid artery is identified at the same level at the depths of the cervical wound. The artery is clamped with fine right-angled clamps, divided, and ligated. The deep cervical fascia overlying the prevertebral fascia is now incised over the length of the cervical incision. The prevertebral fascia is palpated at the depths of the wound, and the superior mediastinum is entered by blunt finger dissection along the prevertebral fascia. As the trachea is retracted medially, the left anterior cervical strap muscles are tensed, elevated with a right-angled clamp near the left clavicle, and divided with electrocautery, thereby facilitating exposure of the esophagus at the thoracic inlet.
the underlying platysma muscle, and the fascia along the anterior edge of the sternocleidomastoid muscle is incised in the direction of the wound. Blunt finger retraction is used to separate the sternocleidomastoid muscle from the underlying cervical fascia medial to it. Retraction of the sternocleidomastoid muscle laterally allows identification of the underlying omohyoid muscle, the central tendon of which is elevated with a right-angled clamp and divided (Fig. 3). Elevation of the medial belly of the divided omohyoid muscle tenses the contiguous omohyoid fascia layer, which is incised in the direction of the wound superiorly and inferiorly. The omohyoid fascia layer leads to the carotid sheath and its contents, which are gently retracted laterally. The larynx and the trachea are simultaneously retracted medially by the assistant’s index finger (Fig. 4). No metal retractor is placed against the tracheoesophageal groove during any portion of the operation to minimize the risk of recurrent laryngeal nerve injury. As the interior jugular vein is retracted laterally, the middle thyroid vein is often identified and should be mobilized, clamped at either end with fine right-angled clamps, divided, and ligated. Using the cricoid cartilage as a landmark, the inferior thyroid artery is identified at the same level at the depths of the cervical wound. The artery is clamped with fine right-angled clamps, divided, and ligated. The deep cervical fascia overlying the prevertebral fascia is now incised over the length of the cervical incision. The prevertebral fascia is palpated at the depths of the wound, and the superior mediastinum is entered by blunt finger dissection along the prevertebral fascia. As the trachea is retracted medially, the left anterior cervical strap muscles are tensed, elevated with a right-angled clamp near the left clavicle, and divided with electrocautery, thereby facilitating exposure of the esophagus at the thoracic inlet.
The first assistant elevates the esophagus out of the superior mediastinum by applying gentle upward traction on the tracheoesophageal groove at the level of the cricoid cartilage (Fig. 5). This maneuver provides a greater length of cervical esophagus in the wound for subsequent mobilization. The tracheoesophageal groove is developed by sharp dissection posterolateral to the recurrent laryngeal nerve, but no active attempt is made to visualize the nerve. The anterior wall of the esophagus is gently mobilized along the length of the incision. By careful blunt dissection, the surgeon’s index finger proceeds medially anterior to the esophagus, which is retracted laterally until the index finger can
palpate the medial edge of the esophagus and the prevertebral fascia behind it.
palpate the medial edge of the esophagus and the prevertebral fascia behind it.
While maintaining upward traction on the tracheoesophageal groove and keeping the left index finger medial to the esophagus and against the prevertebral fascia immediately behind it, a long right-angled clamp is passed posteriorly along the lateral aspect of the esophagus until its tip can be felt by the index finger medially. Once this circumferential mobilization of the esophagus is completed, the esophagus is encircled with a 1-in. rubber drain (Fig. 6). By retracting the rubber drain superiorly, the cervicothoracic esophagus is elevated to the surface of the wound. As the esophagus is elevated, blunt dissection of the esophagus from the superior mediastinum is carried out while keeping the volar aspects of the fingers closely applied to the esophagus (Fig. 7). With the cervical esophagus encircled and several centimeters of the upper thoracic esophagus bluntly mobilized within the superior mediastinum, the cervical dissection is completed.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree