Total Thyroidectomy, Lymph Node Dissection for Cancer
Thomas W.J. Lennard
The normal thyroid gland is composed of two symmetrical lobes lying on either side of the trachea and joined by an isthmus at the level of the second, third, and fourth tracheal rings. The gland lies underneath the strap muscles of the neck (the sternothyroid and the sternohyoid muscles), each lobe on either side of the larynx and trachea. The gland is invested by pretracheal fascia, which is responsible for its movement during swallowing. In some patients, there is an upward extension of the gland from the isthmus called the pyramidal lobe and this represents a developmental island of tissue in the position of the thyroglossal duct. Accessory and separate islands of thyroid tissue can be found in the superior mediastinum near the hyoid bone and beneath the sternomastoid muscle. The thyroid gland descends into the neck embryologically, following a proliferation of the cells at the junction of the anterior third and posterior two thirds of the tongue. The importance of understanding this developmental pathway is essential if a surgeon is trying to remove the whole of the thyroid tissue and on occasion division of the hyoid bone and tracing the thyroglossal duct all the way toward the base of the tongue may be required. In addition, embryologically, the calcitonin-producing cells join the thyroid gland having migrated from the neural crest. Other neural crest tissue forms part of the adrenal glands and the parathyroid glands, and this explains the combination of multiglandular disease in the syndromes known as multiple endocrine neoplasia type II.
The thyroid gland obtains its blood supply through the superior and the inferior thyroid arteries. The superior thyroid artery is the first branch from the anterior aspect of the external carotid artery and it reaches the gland as a single vessel, usually at the upper pole of the thyroid. The inferior thyroid artery, by contrast, divides outside the thyroid gland into four or five branches that pierce the gland supplying the lower pole of it. The recurrent laryngeal nerve lies usually behind the inferior thyroid artery, but it can on occasions lie in front of it or weave its way between the branches of the artery. There is a variable relationship between the external branch of the superior laryngeal nerve and the superior thyroid artery, the most common variable being that the nerve is some distance from the artery, but on occasions it can again weave its way between the branches of the artery and be very close to the upper pole of the thyroid. Because of the close relationship of these two nerves to the blood supply of the thyroid, the technique of capsular dissection of the thyroid, whereby the surgeon stays close to the capsule of the thyroid, will ensure the greatest protection for these important nerves. Very rarely, there can be an inferior artery of the thyroid (the thyroid ima artery), which enters the lower part of the isthmus directly from the brachiocephalic trunk or occasionally from the arch of the aorta.
The venous return from the thyroid in the upper pole follows the superior thyroid artery, but for the middle part of the thyroid, a separate, short and wide vein, usually single but sometimes with several branches, drains directly into the internal jugular vein. During mobilization of the thyroid, this vein or series of veins needs to be secured early before traction is put on the lobe because tearing can cause substantial hemorrhage and make identification of other vital structures nearby extremely difficult. The third group of veins, the inferior thyroid veins, drains the lower poles of the thyroid forming a plexus that runs down into the brachiocephalic veins. Lymphatic drainage of the thyroid follows the arteries, but there is considerable crossover of lymphatic flow between the neck compartments in the presence of malignant disease.
The nerve supply to the thyroid gland is predominantly from the sympathetic cervical ganglion, these fibres being vasoconstrictor.
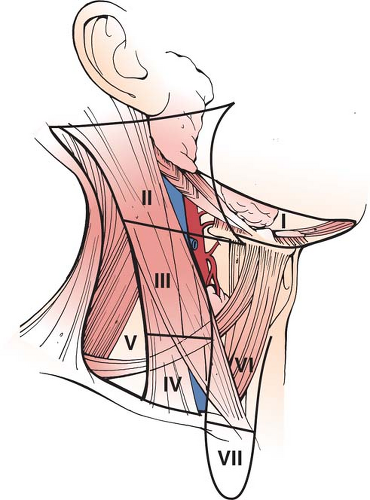
Close to the thyroid gland and intimately associated with it and sharing its blood supply are the parathyroid glands. There are typically four parathyroid glands—two on each side—and in health both glands lie within 1 cm radius of the inferior thyroid artery as they begin to break into its branches to supply the thyroid. The gland situated superiorly is called parathyroid four because it develops from the fourth pharyngeal pouch, and that below the lower pole of the thyroid is called parathyroid three, developing from the third pharyngeal pouch. The anatomical position of the parathyroid glands is somewhat variable and on occasions the glands can be within the thyroid, attached to it or lying along the thyrothymic tract. Preservation of these glands and their blood supply during thyroidectomy is important and parathyroid dysfunction following thyroidectomy is the commonest complication of thyroidectomy. Structures close to the thyroid gland, which become relevant during nodal dissection include the trachea, the esophagus, and the carotid sheath. The latter contains the common carotid artery, the internal jugular vein, and the vagus nerve. A surgeon operating on the thyroid gland for malignancy will need a good understanding of the lymph node territories of the neck (see Fig. 1).
Thyroid cancer presents most commonly as a painless lump within the thyroid gland. Such swellings usually will be >1 cm in size, if clinically palpable. As the tumor growth progresses, compressive symptoms may occur causing dysphagia or dyspnea, and the patient may become aware of supraclavicular swellings in the neck or swellings in the posterior triangle of the neck representing involved lymph nodes. Rarely, thyroid cancer can present as disseminated disease most typically with bone, lung, or mediastinal tumor deposits. Differentiated thyroid cancers (papillary and follicular) are nonsecretory tumors, so there are few, if any, systemic symptoms during tumor development. On the other hand, medullary thyroid cancer arising within the C cells secretes calcitonin, and this can cause systemic symptoms including blushing and diarrhoea. Undifferentiated or anaplastic thyroid cancer may present as a diffuse rapidly growing goiter, with early compressive symptoms due to the infiltrative nature of the disease. If the great vessels of the neck are involved in this process, venous congestion may be clinically evident in the region of the face and the neck.
Increasingly, thyroid cancer is being diagnosed as a result of an incidental finding as a consequence of a radiological investigation unrelated to the thyroid (the so-called thyroid incidentaloma). This is further discussed elsewhere in this chapter. These tumors are commonly <1 cm in size, although
on occasions patients may be harboring large tumors, which they were unaware of and which are picked up on cross-sectional imaging for other reasons. With the advent of genetic testing for multiple endocrine neoplasia type II, patients are now presenting through a family member having been diagnosed with the disease leading to predisposition testing. Depending on the age and specific mutation involved, some of these patients may be diagnosed before their medullary thyroid cancer is developed and is at the stage of C cell hyperplasia. However, when an index case is discovered, inevitably some family members are found who have the established disease and the presentation is through the family history rather than a presenting complaint for the individual concerned.
on occasions patients may be harboring large tumors, which they were unaware of and which are picked up on cross-sectional imaging for other reasons. With the advent of genetic testing for multiple endocrine neoplasia type II, patients are now presenting through a family member having been diagnosed with the disease leading to predisposition testing. Depending on the age and specific mutation involved, some of these patients may be diagnosed before their medullary thyroid cancer is developed and is at the stage of C cell hyperplasia. However, when an index case is discovered, inevitably some family members are found who have the established disease and the presentation is through the family history rather than a presenting complaint for the individual concerned.
The patient presenting with a nodule, thought to be arising within the thyroid, will clearly need a careful history and examination before embarking upon diagnostic tests. In the history, care should be taken to evaluate whether the patient is hyperthyroid, hypothyroid, or euthyroid. In thyroid cancer, the patient most commonly will have neither over nor underactivity of the thyroid. A careful family history should be sought for diseases associated with the thyroid and the development of tumors in candidate organs namely adrenal disease and parathyroid disease in family members. In addition, a personal history of irradiation or exposure to radiation, particularly under the age of 16 should be checked. Familial polyposis and Cowden’s syndrome should be enquired about, the latter being an association between thyroid cancer, macrocephaly, and breast cancer. If the patient gives a history of rapid growth of the thyroid swelling and hoarseness of the voice or symptoms suggestive of disseminated disease such as bone pain, then suspicion of thyroid malignancy is raised. Thyroid nodules occurring in children are more likely to be malignant than in adults and it should be remembered that thyroid cancer presents most commonly in females around the age of 40.
Physical findings will include the swelling in the neck, a careful search for associated lymphadenopathy, an assessment of the voice, and the differentiation of a true thyroid nodule, which moves on swallowing from other nonthyroid swellings in the neck. Baseline blood tests will include tests of hemoglobin, renal function, and liver function, and specifically for the thyroid, thyroid function tests (including TSH, T4, and T3 level) should be performed. A calcium level should also be taken. If the TSH level is low and below the normal range, then a radionuclide thyroid scan should be performed to see if the thyroid nodule is hyperfunctioning. If so, further pathological evaluation is not required since hyperfunctioning nodules are very rarely malignant. However, if the patient has a hyperthyroid state that is not concordant with a hyperfunctioning nodule on radionuclide scanning then the nodule does require separate evaluation by way of biopsy (see below).
A diagnostic thyroid ultrasound should be performed in all patients with a thyroid nodule with the aim of confirming that the nodule is arising within the thyroid and assessing its size. There are some ultrasound features of thyroid nodules, which are more suspicious of malignancy and in addition the lymph nodes in the neck can be assessed for evidence of metastatic involvement. The ultrasound scan can also be used to take a guided biopsy, and nodules that were not palpable and known about may be seen on the scan and can also be biopsied and assessed. There is no clear evidence that suggests that calcitonin should be measured in every patient with a thyroid nodule and although this is a very specific test for medullary thyroid cancer, the measurement of calcitonin as a screening tool is unproven and currently not recommended.
Once a nodule is identified, then a fine-needle aspiration (FNA) biopsy is required. In large solitary nodules, this can be performed in the clinic freehand without the use of ultrasound guidance; however, increasingly, ultrasound guidance of biopsy is employed to
ensure the accurate assessment of the palpable nodule. Difficulties can arise when multiple nodules are detected by ultrasound, and deciding which nodule to sample in this setting can be challenging for the radiologist and/or clinician. Ultrasound features, which are suggestive of malignancy, include a hypoechoic nodule, increased vascularity, irregular margins, the presence of microcalcifications, and hardness of the nodule (elastography). Whilst no one feature is diagnostic, an experienced radiologist will often be able to combine the above features into a sensitive diagnostic probability. When thyroid abnormalities picked up by cross-sectional imaging or ultrasound for other reasons (the incidentaloma) are <1 cm in size, routine FNA biopsy is not recommended unless there are features that are suggestive of malignancy including the presence of lymph nodes nearby, which are concerning. If patients are in a higher risk category (family history, previous radiation) or if the thyroid nodule was picked up through a positron emission tomography (PET) scan, then there will be a greater tendency to perform biopsy on subcentimeter nodules than if this was not the case. In experienced hands, either guided or freehand FNA biopsy of thyroid nodules has a good specificity and sensitivity. However, if the cytopathologist does not have enough cellular material to make a diagnosis, the FNA should be repeated and it should be remembered that a small number of thyroid nodules (<10%), despite repeatedly reported as benign or serial biopsies, will turn out to be malignant when resected. Classification of thyroid FNA biopsy specimens is usually based on a scale of 1 to 5. Thy1 suggests insufficient cells for a diagnosis, Thy 2 is benign, Thy3 indeterminate, Thy4 suspicious of malignancy with around a 95% specificity, and Thy5 a clear case of a malignant diagnosis. Whilst FNA biopsy can accurately diagnose papillary thyroid cancer, medullary thyroid cancer and sometimes anaplastic cancer, it will not differentiate nodules that are due to follicular thyroid cancer or lymphoma. For the latter (lymphoma), a guided core biopsy will often be sufficient and open surgery is seldom required. For the former, follicular neoplasms, diagnostic thyroid lobectomy is the only way currently available to confirm or refute the difference between a benign follicular adenoma and a follicular carcinoma of the thyroid.
ensure the accurate assessment of the palpable nodule. Difficulties can arise when multiple nodules are detected by ultrasound, and deciding which nodule to sample in this setting can be challenging for the radiologist and/or clinician. Ultrasound features, which are suggestive of malignancy, include a hypoechoic nodule, increased vascularity, irregular margins, the presence of microcalcifications, and hardness of the nodule (elastography). Whilst no one feature is diagnostic, an experienced radiologist will often be able to combine the above features into a sensitive diagnostic probability. When thyroid abnormalities picked up by cross-sectional imaging or ultrasound for other reasons (the incidentaloma) are <1 cm in size, routine FNA biopsy is not recommended unless there are features that are suggestive of malignancy including the presence of lymph nodes nearby, which are concerning. If patients are in a higher risk category (family history, previous radiation) or if the thyroid nodule was picked up through a positron emission tomography (PET) scan, then there will be a greater tendency to perform biopsy on subcentimeter nodules than if this was not the case. In experienced hands, either guided or freehand FNA biopsy of thyroid nodules has a good specificity and sensitivity. However, if the cytopathologist does not have enough cellular material to make a diagnosis, the FNA should be repeated and it should be remembered that a small number of thyroid nodules (<10%), despite repeatedly reported as benign or serial biopsies, will turn out to be malignant when resected. Classification of thyroid FNA biopsy specimens is usually based on a scale of 1 to 5. Thy1 suggests insufficient cells for a diagnosis, Thy 2 is benign, Thy3 indeterminate, Thy4 suspicious of malignancy with around a 95% specificity, and Thy5 a clear case of a malignant diagnosis. Whilst FNA biopsy can accurately diagnose papillary thyroid cancer, medullary thyroid cancer and sometimes anaplastic cancer, it will not differentiate nodules that are due to follicular thyroid cancer or lymphoma. For the latter (lymphoma), a guided core biopsy will often be sufficient and open surgery is seldom required. For the former, follicular neoplasms, diagnostic thyroid lobectomy is the only way currently available to confirm or refute the difference between a benign follicular adenoma and a follicular carcinoma of the thyroid.
The use of molecular markers and PET scanning, whilst seeming promising, to help in this conundrum is not accurate enough at the present time to substitute for diagnostic thyroid lobectomy. Because of the previously stated small number of thyroid nodules, which appear benign on ultrasound and FNA biopsy but subsequently turn out to be malignant, follow-up of patients with benign thyroid nodules is advised and repeat biopsy should be considered if the nodule grows in size, either clinically or on ultrasound. There are no clear guidelines for thresholds of nodule growth or timescales for repeat assessments but the American Thyroid Association guidelines in 2009 suggest that a 20% increase in nodule diameter should prompt further biopsies. These biopsies should be performed at between 6 and 18 months after the initial FNA biopsy. If a firm diagnosis of thyroid cancer is made or there is a strong suspicion of this (Thy4 and Thy5), then careful ultrasound evaluation of the loco regional lymph nodes should be performed. Biopsy should be undertaken of any concerning lymph nodes, which may clarify the situation in a patient with Thy4 cytology and will allow appropriate nodal surgery to be planned at the first operation.
The treatment of thyroid cancer and associated involved lymph nodes needs to be carefully thought through and discussed as part of a multidisciplinary approach. It is a potentially lethal disease and must be respected as such. The treatment will often be multimodal including surgery, radio iodine, and TSH suppression with Thyroxine. Careful plans for the postoperative management and monitoring of the patient, including the measurement of thyroglobulin levels, intermittent scans, and clinical follow-up, will all be needed. There are several published guidelines regarding the treatment of thyroid cancer, including those published by the American thyroid Association (2009), the British Thyroid Association, and the British Association of Endocrine and Thyroid Surgeons (2007), together with a recently published evidence-based review of surgery for thyroid cancer published in the World Journal of Surgery (May 2007).
The obvious central strategy for the treatment of the disease is the complete removal of the primary tumor and, wherever possible, any extensions out with the thyroid gland, together with the removal of the relevant involved lymph nodes. Nevertheless, the execution of this relatively simple sounding strategy becomes somewhat more complicated as the individual variables for each patient are considered. Comorbidities in the patient, precise type of thyroid cancer, size of the primary involvement or otherwise of the lymph nodes, particular subtype of tumor together with personal and family history will all influence the final choices and options available to the patient. There is clear evidence that completeness of surgical resection is an important factor in securing a successful outcome, and recurrence within the neck remains the most common site of recurrence. Nevertheless, our understanding of the biology of thyroid cancer, particularly in relation to small primary tumors and small deposits within the lymph nodes, is incomplete. Overtreatment can result in considerable morbidity with no gain to the patient and undertreatment clearly may compromise the final outcome. As already mentioned in the section on “Diagnosis,” accurate preoperative staging of the disease by neck imaging is important. However, preoperative ultrasound does not identify all involved lymph nodes and is somewhat operator dependent. Lymph nodes in the neck are divided into anatomical compartments (see Fig. 1). The level 1 lymph node compartment is the submental and submandibular node compartments above the hyoid bone. Levels 2, 3, and 4 lymph nodes are aligned along the jugular veins on each side between the posterior border of the sterno cleido mastoid muscle and the anterior level 6 compartment. The level 5 nodes are in the posterior triangle lateral to the sterno mastoid muscle and the level 6 nodes are central, running from the hyoid bone down to the suprasternal notch. Superior mediastinal lymph nodes above the level of the innominate artery and in the upper mediastinum are referred to as level 7 nodes. Removal of the thyroid tumor in the neck and associated lymph nodes may well be an appropriate treatment even in the context of disseminated disease, since once the primary and loco regional nodes have been removed, metastases are more easily treated with radioactive iodine in the absence of other tissue avid for iodine. For goiters limited to the neck, ultrasound alone is the imaging modality of choice in planning treatment, but in retrosternal goiters or those where medullary thyroid cancer is thought to be present, CT or cross-sectional imaging of the mediastinum is advised. If the preoperative investigations have fallen short of a firm diagnosis of established thyroid cancer, then a diagnostic hemithyroidectomy is required on the affected side. Wherever possible, surgeons should try to remove all the thyroid tissues on the affected side at the time of a diagnostic lobectomy, so that further surgery is not required in that compartment. Revisional surgery to remove a remnant of thyroid tissue following an incomplete lobectomy is dangerous for the patient in terms of risks to the recurrent laryngeal nerve and parathyroid gland, as well as challenging and difficult for the surgeon. If a patient has a suspicious FNA biopsy and other features, which make it likely that they have a thyroid cancer in their history, or comorbidities which would make a second operation dangerous if the diagnostic lobectomy prove to be malignant, then a discussion can take place with the patient about the validity of proceeding immediately to a total thyroidectomy, even when the diagnosis falls short of confirmed thyroid cancer. This may be particularly relevant when the patient has a multinodular goiter, which in its own right, even if it is benign, might require total thyroidectomy. Because of multifocality in thyroid cancer of all types, if the primary tumor is >1 cm in size, then total thyroidectomy is the operation of choice. For a small subcentimeter low-risk thyroid cancer with no predisposing risks for further disease (e.g., radiation or family history) or evidence of disseminated disease (enlarged lymph nodes on ultrasound), a unilateral lobectomy may be sufficient. Nevertheless, the patient should be counselled that although metastasis and multifocality are unlikely with subcentimeter differentiated thyroid cancers, follow-up by using thyroglobulin measurements in patients with residual thyroid tissue and imaging the thyroid gland with radionuclide scans is more difficult when a normal lobe or part of a normal lobe has been left in situ.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree