Total Proctectomy with Sphincter Preservation for Distal Rectal Center
James W. Fleshman
Bashar Safar
Nearly 40,000 patients are diagnosed with rectal cancer annually in the United States. Despite a recent decrease in mortality for colorectal cancer, it remains the second leading cause of cancer deaths in the United States. This chapter addresses cancers of the distal rectum. Rectal cancer management differs in many ways from colon cancer. The distal rectum is confined by the bony pelvis making surgical dissection more challenging particularly in large distal tumors. Local recurrence in the pelvic is an extremely distressing condition for patients to live with or die from and represents a measurable end point of therapy in distal rectal cancer. Rectal cancer is a surgical disease; sound knowledge of the surgical anatomy and adherence to oncologic principles in combination with radiation therapy minimizes local recurrence.
Anatomy
The rectum is defined as the terminal part of the large intestine. It extends from the anal verge to the pelvic brim. The measurement
between the anal verge and the pelvic brim may vary due to variation in anal canal length; however, the rectum has arbitrarily between designated to be the 12 cm above the anal verge found at the intersphincteric groove in the distal anus (JNCI-Nelson 1999) (Fig. 1). The rectum is divided into three segments for descriptive purposes: lower, middle, and upper. Each segment measures roughly 4 cm in length, divided by two curves, which in turn give rise to the two valves separating the rectal segments. Tumor location is usually described as distance from anal verge but may also be measured from the dental line. In addition, tumor location can be described in relation to rectal valves.
between the anal verge and the pelvic brim may vary due to variation in anal canal length; however, the rectum has arbitrarily between designated to be the 12 cm above the anal verge found at the intersphincteric groove in the distal anus (JNCI-Nelson 1999) (Fig. 1). The rectum is divided into three segments for descriptive purposes: lower, middle, and upper. Each segment measures roughly 4 cm in length, divided by two curves, which in turn give rise to the two valves separating the rectal segments. Tumor location is usually described as distance from anal verge but may also be measured from the dental line. In addition, tumor location can be described in relation to rectal valves.
The posterior rectum is an entirely extraperitoneal structure surrounded by the mesorectum. Anteriorly, the rectum is extraperitoneal in the lower one-third only. A thin layer of investing fascia (fascia propria) coats the mesorectum and represents a distinct layer from the presacral fascia against which it lies. The visceral and parietal fascias are separated by a layer of avascular areolar tissue, which guides posterior dissection during proctectomy. The mesorectum contains the superior hemorrhoidal artery along with draining veins and lymph nodes. The rectosacral fascia, or Waldeyer’s fascia, is a thick condensation of endopelvic fascia posteriorly connecting the presacral fascia to the fascia propria at the level of S4 and extends to the anorectal ring (Fig. 2). Waldeyer’s fascia is an important surgical landmark, and its division during dissection from an abdominal approach provides entry to the deep retrorectal pelvis. The anterior fascial covering of the mesorectum is referred to as Denonvilliers’ fascia.
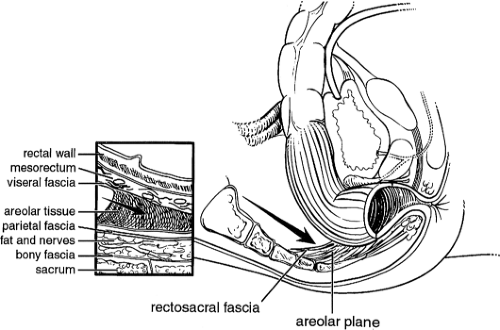 Fig. 2. The rectosacral fascia must be sharply divided to allow full mobilization past the coccyx to the level of the anorectal ring. |
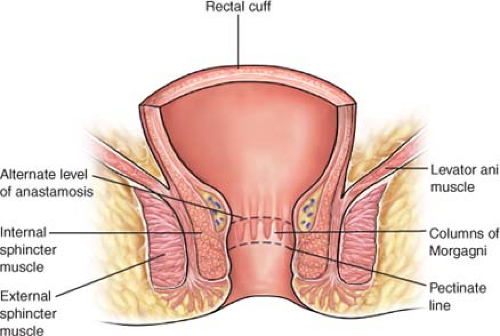
The rectum terminates at the anorectal junction, which corresponds to the levator ani muscle. The levator ani muscle is a continuation of the external sphincter muscle. Preservation of both of these muscles is vital for adequate continence and acceptable quality of life following low rectal procedures.
Autonomic Nervous System
The parasympathetic nervous system supplies branches to the rectum from the second to fourth sacral nerves via the nervi erigentes. These nerves enter the pelvis through the sacral foramina where they are covered by parietal fascia. The sympathetic nervous system supplying the pelvis arises from splanchnic branches of T12-L2, which forms a plexus at the takeoff of the inferior mesenteric artery (IMA). The plexus is composed of the inferior mesenteric plexus superiorly and the superior hypogastric plexus inferiorly. The hypogastric (parasympathetic and sympathetic) nerves travel caudally down to the sacral promontory where they can be found overlying the parietal fascia and coalesce about 1 cm on either side of the midline. The pelvic splanchnic nerves then travel medial to the ureter along the lateral pelvic walls. Here, the hypogastric nerves converge with the nervi erigentes. These nerves form a trunk of nervous tissue extending from the deep pelvic sidewall, entering the mesorectum between the 10 and 2 o’clock positions along with the internal iliac-derived interior rectal vessels, constituting the anterolateral ligament.
The parasympathetic nervous system is responsible for increased blood flow to the penis (erection), vaginal lubrication, and innervation of the detrusor muscle, whereas the sympathetic system is responsible for emission, orgasm, and inhibition of the detrusor muscle. Injury to the autonomic nervous system results in impotency, bladder dysfunction, or both.
Vascular Supply
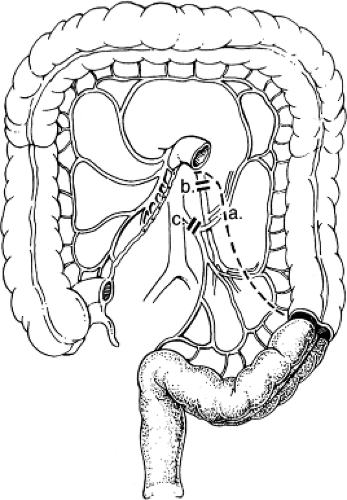
The rectum receives blood from three sources (Fig. 3). The IMA bifurcates at the base of the sigmoid mesentery to become the left colic and superior rectal arteries. The superior rectal artery descends into the pelvis in the mesorectal fat and divides again into the right and left superior rectal arteries. These vessels accept vessels carrying blood from the middle rectal arteries, which branches from the internal iliac arteries found at the deep pelvis lateral side wall and come through the anterolateral
ligaments of the rectum. The inferior rectal arteries are branches of the external ileac system via the pudendal vessels on each side of the anal canal and collateralize with the middle rectal arteries in the low rectal wall. These three collateral systems provide an excellent redundant blood supply, which allows routine resection and reanastomosis at almost any level in the pelvis, even after ligating the IMA at is origin.
ligaments of the rectum. The inferior rectal arteries are branches of the external ileac system via the pudendal vessels on each side of the anal canal and collateralize with the middle rectal arteries in the low rectal wall. These three collateral systems provide an excellent redundant blood supply, which allows routine resection and reanastomosis at almost any level in the pelvis, even after ligating the IMA at is origin.
Early tumors may be completely asymptomatic. They frequently present with rectal bleeding, which is often mistaken for hemorrhoidal bleeding. With advancing local growth, patients may complain of constipation, a change in bowel habit with a change in stool caliber leading up to the large-bowel obstruction. In larger tumors, pain may be a prominent feature and should raise concern for tumor invasion into adjacent organs or the pelvic floor. Distal tumors involving the sphincter complex may present with incontinence.
Patient with rectal cancer may be identified during screening for colorectal cancer. Patients presenting with newly made diagnosis of rectal cancer should undergo the following assessment:
Complete history and physical examination. Thorough assessment of the patient’s functional status and suitability for surgical intervention should be made at the time of initial consultation, as this could help guide future therapy decisions.
A baseline carcinoembryonic antigen (CEA) should be obtained.
Digital rectal exam must be performed on all patients suspected of having rectal cancer to assess the distance of the tumor from the anal verge, fixation or sphincter involvement, and resectability. No accurate information is obtained regarding early T stage (T1 vs. T2) or microscopic lymph node involvement. Eliciting a thorough continence history from the patient and sphincter tone on digital exam alerts the surgeon to any preexisting continence deficits, which may preclude the patient from having a coloanal anastomosis. Patients presenting with preexisting incontinence have unacceptable function after ultralow colorectal anastomosis and should be counseled accordingly.
Rigid proctoscopy can be performed in the office setting and accurately defines the distance of the tumor from the anal verge. Distance measurements using a flexible proctoscope are less accurate than a straight proctoscope.
Colonoscopic examination of the entire colon is essential if the tumor is not obstructing in order to exclude synchronous tumors. Synchronous tumors are defined as two or more distinct primary tumors separated by normal bowel and not due to direct extension of the tumor or metastasis and occur in 3% to 5% of patients with colorectal cancer. In patients whom colonoscopy cannot be completed either due to obstruction or technical difficulties, a double contrast water-soluble enema or a colonography should be performed.
Local Staging
Management decisions are greatly influenced by local staging in rectal cancer. Due to location and ease of access, tumors in the rectum lend themselves to assessment by a number of methods. The goal is to ascertain the depth of invasion in relation to the rectal wall (T stage) and lymph node involvement (N stage).
T stage: Transrectal ultrasound and high-resolution magnetic resonance imaging (MRI) are the most accurate tools in predicting T stage in rectal cancers. In the United States, endorectal ultrasound (ERUS) is most commonly used, whereas in many European countries, high-resolution MRI is preferred. High-resolution MRI reveals deep tumor invasion with encroachment on the mesorectal envelope indication potentially close radial margins after resection. These patients are selected to receive preoperative therapy. The overall accuracy in predicting T stage is approximately 70% to 90% with ultrasound or high-resolution MRI and 50% to 70% with computed tomography (CT) or conventional MR.
N stage: Identification of positive lymph nodes is more difficult. The overall accuracy in detecting positive pelvic lymph nodes with ERUS and MRI outlined is approximately 50% to 75%. Both CT and MRI can identify lymph nodes measuring 1 cm or greater, although enlarged lymph nodes are not pathognomonic of tumor involvement. The accuracy of ERUS for the detection of involved perirectal lymph nodes may be augmented if combined with fine needle aspiration and three-dimensional imaging.
Systemic Staging
A baseline CT scan of the chest, abdomen, and pelvis should be obtained to exclude systemic disease. PET scan is not indicated as a baseline test in the absence of metastatic disease. Please refer to Table 1 for more detail description of tumor staging.
Management of patients with rectal cancer depends on the stage of disease at presentation. Surgical therapy is the cornerstone of curative therapy in rectal cancer. However, patients with stage IV disease may require systemic therapy as the initial therapy and subsequently may never undergo a resection, depending on the extent of the disease and response to systemic chemotherapy. Patients with locally advanced tumors should undergo neoadjuvant therapy aimed at reducing local recurrence.
Preoperative Radiation Therapy
There are two approaches to radiation therapy for rectal cancer. The first approach is the short course radiation therapy only (25 Gy in five fractions with immediate operation). This approach is used most commonly in Europe. The other approach utilizes radiation therapy in combination with chemotherapy with a 6- to 8-week period of rest before operation and is the standard approach in North America. The total radiation dose is 50.4 Gy in 28 fractions. The long course combined chemoradiotherapy approach has the advantage of tumor shrinkage and downstaging of the tumor.
Patients with bulky tumors in close proximity to the sphincters may achieve resectability with sphincter preservation with this approach.
Patients with bulky tumors in close proximity to the sphincters may achieve resectability with sphincter preservation with this approach.
Table 1 Staging of Rectal Cancer | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Distance from Anal Verge
Tumors of the mid and distal rectum have been traditionally treated with an abdominoperineal resection (APR). This was largely based on a need for a 5 cm distal margin for tumor clearance and the high likelihood of local recurrence in low rectal cancers. The adoption of the total mesorectal excision (TME) and neoadjuvant chemoradiation has lead to a decreased local recurrence rates and enabled surgeons to perform restorative procedures in very low tumors. Recent evidence has called into question the need for even a 1 cm distal margin as long as the margin is clear on frozen section intraoperatively. When the distal margin is clear, local recurrence remains acceptably low. This allows for a restorative proctectomy for tumors as low as 2 to 3 cm from anal verge provided the sphincter complex is spared.
Radial margin positivity on the other hand may influence local recurrence to a larger extent than previously appreciated. APRs have been shown to have a higher rate of local recurrence when compared with low anterior resection. This could be influenced by the fact that more tumors that involve the sphincter complex require an APR. In a standard APR, coning at the level of the levator muscles results in the so-called hourglass specimen. This effort is to avoid a large pelvic floor defect that has been associated with higher positive radial margins and local recurrence.
Timing of Surgery
Based on the modality chosen for preoperative neoadjuvant therapy, the patient must be prepared for a major operation. Short course radiation therapy is usually followed immediately (within 1 to 2 days) by surgery. Patients receiving standard chemoradiation should have a hiatus of 6 to 8 weeks prior to surgical intervention. Waiting allows for maximal effect of therapy and resolution of the inflammatory response to radiation, without adversely affecting resectability. Due to the radiation effect, the tissues are edematous at this stage and allow for a relatively noncomplicated pelvic dissection. Standard neoadjuvant therapy may not be well tolerated by some patients, resulting in abbreviation of treatment, and their suitability for a resection should be reassessed at the end of therapy. Routine repeat examination, including repeat ultrasound has not been shown to aid in the assessment of resectability.
Medical Clearance
Patient’s fitness for surgery should be determined based on acceptable guidelines such as American College of Cardiology/American Heart Association (ACC/AHA) or the local equivalent of these guidelines. Laboratory tests should include a complete blood count, electrolytes, and assessment of renal function. A type and screen should be made available on the day of surgery.
Enterostomal Therapy
Temporary diverting ileostomy should be constructed to protect the coloanal anastomosis; especially following radiation therapy. Consultation with an enterostomal therapist prior to surgery helps alleviate many of the patient’s fears regarding stomas. In addition to preoperative counseling, enterostomal therapy (ET) nurses can determine the best stoma location at the same visit and mark the site for the surgeon. Differences in abdominal wall contour, body habitus, and location of belt placement can greatly influence ease of pouching and acceptability of stomas by patients. Proper stoma location should be individualized to each patient and marked with the patient in different positions—laying, sitting, and standing.
Bowel Preparation
Two large meta-analyses have been published recently questioning the need for bowel preparation in colon and rectal surgery. Despite no differences shown in leak rate and postoperative complications, we advocate (per the ASCRS practice parameters) the use of full mechanical bowel preparation in low rectal cancer surgery. Low rectal cancer surgery differs from colon surgery in a number of ways. Coloanal anastomosis has a higher leak rate (10% to 15%) than colonic anastomoses (4%). This rate increases further with the addition of radiation therapy for local disease control. Lack of preparation may convert subclinical leaks that can be treated with percutaneous drains into a clinical leak. Clinical leaks very low in the pelvis may necessitate takedown of the anastomosis with slim to no likelihood of future reversal and carry a high risk of mortality. Moreover, pelvic sepsis following low pelvic surgery results in poor long-term function and may lead to significant morbidity. Considering all the reasons mentioned above, we emphasize nonselective diversion of low coloanal anastomoses with full mechanical bowel preparation.
Obstructing Tumors
Partially obstructing tumors rarely require diversion of stenting prior to initiation of neoadjuvant chemoradiation. A small proportion of patients require intervention and interruption of therapy due to progression of the tumor and closure of the lumen. Neoadjuvant therapy allows the tumor to be downsized and for the obstruction to resolve prior to surgery. If the tumor is completely obstructing, proximal diversion or
stent placement should be considered. Stents need to have a distal landing zone of a few centimeters and cannot be placed in very distal tumors. Both the above strategies allow the completion of neoadjuvant therapy and improved oncologic outcome. Large tumors causing obstruction in the distal rectum should strongly be considered for an APR and laparoscopic placement of a loop colostomy does not prevent a change to sphincter sparing surgery if the tumor responds well to neoadjuvant therapy.
stent placement should be considered. Stents need to have a distal landing zone of a few centimeters and cannot be placed in very distal tumors. Both the above strategies allow the completion of neoadjuvant therapy and improved oncologic outcome. Large tumors causing obstruction in the distal rectum should strongly be considered for an APR and laparoscopic placement of a loop colostomy does not prevent a change to sphincter sparing surgery if the tumor responds well to neoadjuvant therapy.
Specialized Equipment
Deep pelvic dissection is challenging due to the bony confines of the pelvis and difficult visualization. Blunt dissection must be avoided to minimize tumor spillage. A good surgical headlight is helpful for proper visualization of the deep pelvis and is strongly recommended. A self-retaining retractor system is helpful and specialized pelvic retractors such as the lighted narrow St. Marks or Thorlackson retractors are essential tools of the trade. Many publications have confirmed the superior results obtained by subspecialization and high-volume centers. Rectal cancer surgery should be performed by surgeons who specialize in the field of colon and rectal surgery in centers that are well equipped for these procedures.
Extra long instruments (right-angled clamp, DeBakey Forceps, Pean Clamps), extensions to the cautery, long (54 in.) suture, on a CT needle heavy Jones decapitation scissors, a 35-mm TA stapler, Thorlakson Transverse atraumatic bowel clamp, and the large DASH rectractor have all been helpful in the deep pelvis and difficult cases.