Total Gastrectomy for Carcinoma
Murray F. Brennan
Complete operative resection remains the only potentially curative modality for gastric adenocarcinoma. The results of resection for early gastric cancer are excellent. The majority of patients in the United States present with symptomatic advanced lesions, but earlier lesions are being seen. In 2005 to 2008, 59% of patients operated upon at Memorial Sloan Kettering Cancer Center were T1 to T2 compared with 30% in 1985 to 1988. Operative decisions focus on the most effective procedure that offers potential for cure or on how to achieve the maximal palliation with the minimal morbidity.
When considering a patient for gastric resection for adenocarcinoma several questions must be asked: Is the patient fit for an operation? Is the operation likely to help? If the operation is to proceed, what is the extent of the gastric resection? What is the extent of the nodal dissection? Is there any value in extended organ resection? Is there an indication for adjuvant therapy, and if so, pre-, intra-, or postoperative?
Many patients who have advanced gastric adenocarcinoma may not be medically fit for any procedure. More difficult, however, is the patient who can tolerate a procedure and has minimal symptoms, but by preoperative studies (e.g., computed tomography (CT) or laparoscopy) has incurable disease (e.g., ascites, peritoneal extension, liver metastases, or positive cytology in the absence of metastatic disease). Such patients should be strongly considered for nonoperative treatment. This chapter will focus on the technical issues of resection, when indicated.
Extent of Gastric Resection
The extent of gastric resection for adenocarcinoma of the stomach is mainly predetermined by the site and extent of the primary neoplasm. Total gastrectomy as a routine procedure for gastric adenocarcinoma has not been shown to improve survival, although this continues to be debated. For those patients in whom adequate (4 to 6 cm) margins beyond the lesion can be obtained, a more limited gastric resection (e.g., proximal esophagogastrectomy or distal subtotal gastrectomy) provides the same survival result for the patient and diminishes early perioperative morbidity. The extent of the margin is rarely the limiting factor in survival. Patients rarely die of local marginal recurrence only, and, similarly, patients who are likely to have positive resection margins are usually those who have large penetrating (T3) or node-positive lesions. The need for a total gastrectomy, however, to encompass all the disease within the stomach should never be a factor in precluding proceeding with the operation. The decision in favor of total gastrectomy for proximal lesions is based on long-term sequelae of increased symptomatic reflux when proximal gastrectomy is performed.
Extent of Lymph Node Dissection
The early involvement of lymph nodes is predicated on the site of the primary lesion within the stomach. With the advent of a more reproducible staging system, a minimum of 16 lymph nodes should be resected and identified for adequate staging.
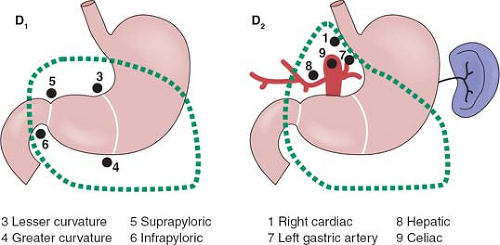
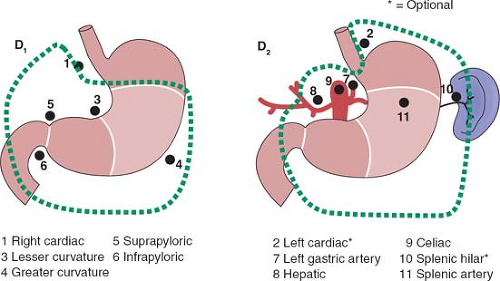
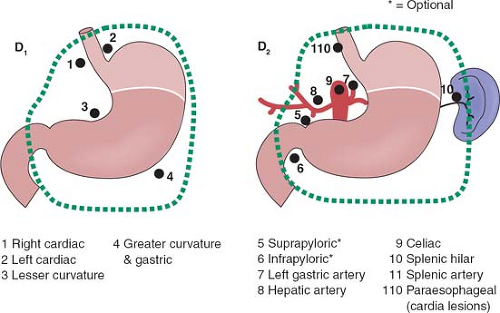
The rationale for more extensive nodal dissection continues to be a matter of great debate. Early studies in Western centers suggested a limited advantage to the more extensive operation. This, however, was neglected as an approach until popularized by the Japanese with reported improved survival results and no greater morbidity. The improved survival results seen by the Japanese authors have been suggested to be due, in part, to the more extended nodal dissection. Whether this is true or due to more adequate and appropriate staging remains a matter of controversy. It would seem that biologically, although early forms of the disease are commonly seen in Japan, the nature of the underlying disease process is not different between Asian and Western populations. The extent of dissection required for a D1 and D2 dissection based on site of the primary is outlined in Figures 1 to 3.
A randomized trial has been completed by the Dutch in which no survival benefit to extended node dissection was found. The patients who had extended node dissection, especially those undergoing pancreaticosplenectomy, had increased morbidity and mortality. The strengths and weaknesses of this trial have been highlighted. As extended node dissection can be performed by experienced hands with only limited morbidity, experienced centers with a large volume continue to use extended node dissection but usually without distal pancreatectomy or splenectomy, if only to gain more accurate staging and for the possibility of improving survival in selected subgroups, such as Stage IIIA and IIIB.
It is important to emphasize the change in the staging system with a requirement of a minimum of 16 nodes to be identified for accurate staging. Nodal staging is then characterized according to the number of positive nodes (PNs), with PN1 reflecting one to two PNs; PN2, three to six PNs; and PN3, more than seven PNs.
Use of Extended Organ Resection
Any extended resection, whether of the spleen, pancreas, colon, or a major artery, is accompanied by increased morbidity and mortality with no improvement in survival.
Local organ resection, especially of the spleen, pancreas, or mesocolon, should be reserved for those lesions, especially N0, in which it is required for complete local resection of the primary tumor.
Local organ resection, especially of the spleen, pancreas, or mesocolon, should be reserved for those lesions, especially N0, in which it is required for complete local resection of the primary tumor.
Operative Preparation
Total gastrectomy remains a major operation in the hands of all but the experienced gastric surgeon. It is our practice to perform a total gastric resection when 4 to 6 cm of negative margins cannot be obtained from the primary tumor. We believe an extended dissection (D2) is the nodal dissection of choice. In all centers a minimum of 16 nodes should be identified and sampled for accurate staging.
Evaluation of the patient who has suspected or proven gastric adenocarcinoma is dominated by the use of endoscopic techniques, with endoscopic ultrasound. Endoscopy allows the definition of the extent of the lesion within the stomach, along with confirmation of the histopathologic diagnosis. Endoscopic ultrasound allows greater definition of the extent of tumor invasion (T stage) and is the most accurate method for defining T stage. Although endoscopic ultrasound provides information on the size of perigastric lymph nodes and can suggest nodal involvement, it is not definitive. Small amounts of ascites, often unappreciated by other studies, can be seen in the lesser sac with endoscopic ultrasound, and unsuspected small liver metastases in the left lateral segment can be identified, but are rare.
CT remains the only other study of value in defining extent of disease noninvasively. Although not as accurate as endoscopic ultrasound for defining T stage, it is more accurate for defining M stage (metastasis) and for detecting ascites and peritoneal or omental deposits. Limited experience has been reported with the use of positron emission tomography scans to determine sites of unexpected metastasis. Unfortunately flcurodeoxyglucose-positron emission tomography (FDG-PET) has limited value except in gastroesophageal junction tumors. With preoperative staging of this nature, consideration as to curative versus noncurative operation can be embarked on. Many surgeons use laparoscopy to further determine the possibility of curative resection. Many patients who have disease unresectable for cure can avoid an unnecessary and nonpalliative extensive operation by the use of laparoscopy to identify unsuspected sites of metastasis, especially peritoneal or hepatic, which occur in up to 20% of radiologically resectable tumors. On occasion, especially for distal lesions, palliative operations to relieve obstruction or bleeding are justifiable; however, significant bleeding is rare in conventional gastric adenocarcinoma. Peritoneal cytology obtained at the time of laparoscopy provides additional prognostic information whether by conventional cytology, cytology with immunohistochemistry (IHC), or reverse transcriptase polymerase chain reaction (RT-PCR). The presence of cytology-positive peritoneal washings even in the absence of visible peritoneal metastases augurs for a prognosis similar to M1 disease.
Patients who have undergone endoscopic ultrasound and laparoscopy and have been shown to have advanced T3 lesions apparently confined to the stomach can be considered for preoperative chemotherapy. This therapy can be delivered safely but with some increase in perioperative morbidity. Given the (5-year) survival of most T3 or N1 lesions of less than 50% and the benefit of preoperative therapy confirmed by randomized controlled trials, this should be the preferred approach unless contraindicated by logistics or comorbidity.
The patient is evaluated for tolerance for a potential major upper abdominal operation. If the gastroesophageal junction is involved and there is a likelihood that a thoracic approach will be required, preoperative pulmonary function may be necessary. Preoperative antibiotics, usually with a cephalosporin, are used at the time of induction, generally as a single dose, dependent on the half-life of the drug used and the length of the operation, with the intent of diminishing wound infection.
Positioning
The patient is placed supine, with consideration given to the possibility of left or right thoracic approach in proximal gastroesophageal lesions. In both positions, the chest is prepared before the patient is allowed to fall back onto a pneumatic mattress, so that repreparation and redraping of the chest can be avoided. A sandbag can be placed beneath the left costal margin to elevate it, and the lower left chest prepared for an extension directly into the left chest. A split-lumen endotracheal tube is placed in all patients in whom thoracotomy is likely. In the majority of patients undergoing total gastrectomy, the lesion is confined to the stomach and an abdominal approach is adequate.
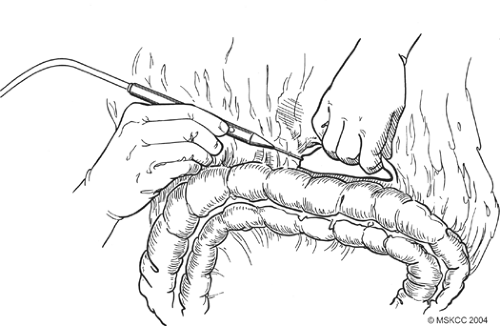 Fig. 4. Detachment of the greater omentum from the colon through the avascular plane using the cautery. |
There is no clear consensus as to whether a long midline incision or a bilateral subcostal incision is preferable. Both provide excellent exposure to the upper abdomen. We tend to use the midline incision when a thoracic extension or second incision is considered, but either approach is acceptable. An alternative approach, a left thoracoabdominal incision, is preferred by some for gastroesophageal junction lesions, but results in greater perioperative morbidity.
We prefer laparoscopy preoperatively to exclude unsuspected metastatic disease. Once access to the abdomen is obtained, careful examination for the extent of disease is performed. Important considerations are the presence of peritoneal metastasis or liver involvement. Remote lymph node involvement (e.g., in the para-aortic lymph nodes) precludes proceeding to an extended node dissection. Lesions involving all of the stomach, but predominantly the proximal stomach with extension to the lymph nodes high in the porta, also preclude the likelihood of any chance for curative resection.
We prefer laparoscopy preoperatively to exclude unsuspected metastatic disease. Once access to the abdomen is obtained, careful examination for the extent of disease is performed. Important considerations are the presence of peritoneal metastasis or liver involvement. Remote lymph node involvement (e.g., in the para-aortic lymph nodes) precludes proceeding to an extended node dissection. Lesions involving all of the stomach, but predominantly the proximal stomach with extension to the lymph nodes high in the porta, also preclude the likelihood of any chance for curative resection.
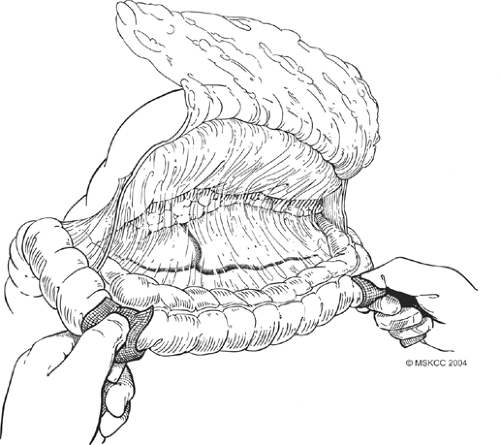 Fig. 5. The anterior layer of the mesocolon is sharply dissected from the mesocolonic vessels in an avascular plane. |
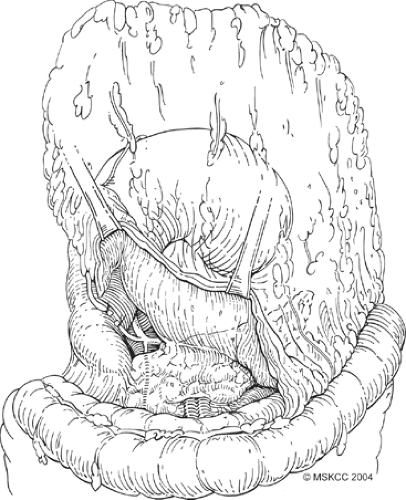 Fig. 6. Careful ligation of the gastroepiploic vessels is essential to avoid an annoying hemorrhage. |
Once formal laparotomy has been used, fixed retractors are placed. Our first approach in the dissection is the section of the greater omentum from the colon (Fig. 4). This is performed by using the cautery and entering into the anterior leaf of the mesocolon. It can take a small amount of time to obtain the correct plane and to skeletonize mesocolonic vessels. Once the correct plane is reached, bleeding should be minimal, and the presence of any hemorrhage suggests an incorrect plane. The splenic flexure of the colon is fully mobilized inferiorly. On occasion, local invasion or adherence is present and mesocolonic resection is required. The standard dissection is continued back to the inferior border of the pancreas, and the pancreatic capsule is dissected upward (Fig. 5). Branches to the right gastroepiploic vessels are divided just at the inferior border of the pancreas, and venous tributaries here should be carefully divided to prevent annoying hemorrhage (Fig. 6). It is unclear that taking the pancreatic capsule improves the quality of the procedure, and as it can result in pancreatic juice leakage if done incorrectly, it may be omitted. The extension is continued out laterally, along with the superior aspect of the pancreas, skeletonizing the splenic
artery (Fig. 7) and dividing the short gastric vessels close to the spleen (Fig. 8). At this point it is often easier to change the approach and begin by dissecting the lesser omentum from the undersurface of the liver, extending back to the right crus and mobilizing the right aspect of the gastroesophageal junction.
artery (Fig. 7) and dividing the short gastric vessels close to the spleen (Fig. 8). At this point it is often easier to change the approach and begin by dissecting the lesser omentum from the undersurface of the liver, extending back to the right crus and mobilizing the right aspect of the gastroesophageal junction.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree