Total Gastrectomy for Cancer
Vikas Dudeja
Eugene A. Choi
Waddah B. Al-Refaie
DEFINITION
Total gastrectomy is removal of the stomach in its entirety including the gastroesophageal (GE) junction. This is typically performed for patients with proximal gastric cancer, including Siewert type II and III GE junction cancers,1 in whom a subtotal gastrectomy with 5- to 6-cm proximal margin does not leave a reasonable gastric remnant. Rarely, diffuse involvement of stomach with malignant processes other than gastric adenocarcinoma may warrant a total gastrectomy. Even though proximal gastric resection is widely performed in Asia and select U.S. cancer centers for proximal gastric tumors, this procedure has not gained wide adoption in western centers.2
PATIENT HISTORY AND PHYSICAL FINDINGS
Presentation: In the United States, most patients with gastric cancer are diagnosed at advanced stages. Weight loss, anorexia, dyspepsia, progressive dysphagia, and early satiety are the most common symptoms. Unfortunately, some of these symptoms (dyspepsia, nausea, anorexia) overlap with those of benign reflux disease and explain the late presentation of proximal gastric cancers in the United States. Poor oral intake because of cancer-induced anorexia and dysphagia contributes to patients’ weight loss. As such, patients with more than 10% weight loss are at increased risk of perioperative complications. Thus, quantification of weight loss in the preoperative period provides a meaningful estimate of nutritional status and helps in pretherapy planning in terms of preoperative nutrition. Patients may also present with iron deficiency anemia due to occult blood loss.
Evaluation of the performance status and frailty is important for the formulation of a treatment plan. Eastern Cooperative Oncology Group’s (ECOG) performance status scale provides a standardized method to assess and compare performance status of the patient.3 Patients with ECOG performance score of 0 to 1 are generally able to tolerate major oncologic resections. Those with a score of 2 may require individualized decision making, factoring comorbidities into the overall treatment plan. Patients with a score of 3 and above are unable to tolerate any major oncologic resection. Attention to pretherapy performance status and frailty are critical, as these two important yet underassessed parameters correlate well with the ability to tolerate various oncologic therapies, including major oncologic resection and systemic therapy.
Physical findings: In early stages, the physical examination is essentially normal. However, surgeons need to look for signs of malnutrition. Patients with advanced-stage gastric cancer may present with supraclavicular lymphadenopathy, pleural effusion, abdominal mass, hepatomegaly, malignant ascites, or drop metastases in the form of “Blumer’s shelf” appreciated on rectal examination. Presences of any of these physical findings suggest unresectability.
IMAGING AND OTHER DIAGNOSTIC STUDIES
Laboratory tests: Baseline hemoglobin, platelet count, and complete metabolic profile should be obtained. Serum albumin and prealbumin are useful to guide assessment of nutritional status.
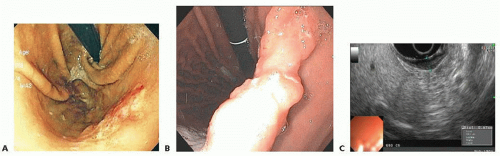
Diagnostic studies and staging evaluation: The evaluation of any upper gastrointestinal symptomatology starts with an esophagogastroduodenoscopy (EGD), especially if gastric cancer is suspected. Endoscopy provides histopathologic diagnosis as well as gives information on location and extent of gastric tumor, GE junction involvement, presence of linitis plastica, and status of duodenal bulb (FIG 1A,B). Endoscopic
ultrasound (EUS), performed at the time of or subsequent to diagnostic endoscopy, provides the most accurate estimation of the tumor depth (T stage) (FIG 1C) and can help in evaluation and needle biopsy of surrounding lymph nodes.4 Furthermore, EUS provides information on the degree of distal esophageal involvement.
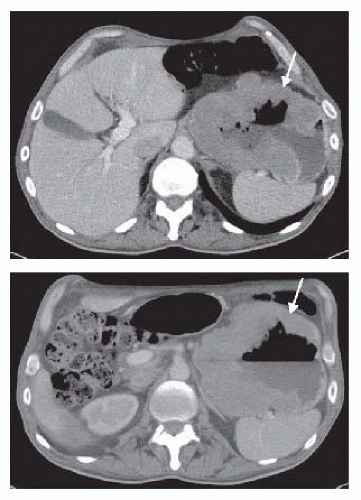
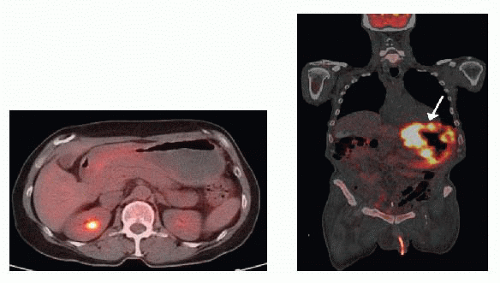
Cross-sectional imaging of abdomen and pelvis, in the form of a computed tomography (CT) scan (FIG 2) with oral and intravenous (IV) contrast or magnetic resonance imaging (MRI), evaluates for presence of distant metastatic disease to the liver and bulky lymphadenopathy. Metastases to lungs should be evaluated by CT scan of the chest. The presence of bulky adenopathy has a prognostic value. However, it should not preclude resection unless patient presents with diffuse adenopathy involving the periportal area or mesenteric vessels. Positron emission tomography (PET)/CT scan has evolved as an additional radiographic staging modality to exclude the presence of distant metastatic disease (FIG 3).
Staging laparoscopy: Peritoneal spread is part of the natural history of gastric cancer. Up to a third of the patients who have localized disease on staging evaluation have unsuspected hepatic and/or peritoneal disease.5 Thus, staging laparoscopy can prevent a nontherapeutic laparotomy in the setting of M1 disease. The timing of staging laparoscopy may depend on the institutional preference with respect to neoadjuvant versus adjuvant therapy. We recommend neoadjuvant chemotherapy for patients with proximal gastric tumors and perform staging laparoscopy (with peritoneal cytology) before initiation of neoadjuvant therapy. We selectively place feeding jejunostomy during staging laparoscopy, especially in persons who are old, frail, have suboptimal performance status, or present with malnutrition. The patient then returns after completion of neoadjuvant chemotherapy for restaging and definitive surgical treatment (i.e., gastrectomy).
Peritoneal cytology: The addition of peritoneal washing for cytology is an area of debate.6,7 We use this diagnostic modality in patients at risk of undeclared metastatic disease or suboptimal performance status, as patients with positive peritoneal cytology have unfavorable overall prognosis compared to those with negative peritoneal cytology.
SURGICAL MANAGEMENT
A complete margin-negative resection with an adequate lymphadenectomy is the most critical component of therapy for operable gastric cancer.
Preoperative Planning
Addressing preoperative malnutrition: Patients with proximal gastric cancer are at an increased risk of being nutritionally compromised due to cancer-induced anorexia and dysphagia. These patients benefit from preoperative enteral nutrition through a jejunostomy tube placed preoperatively during the staging laparoscopy. The enteral nutrition through jejunostomy tube also helps with hydration and nutrition during neoadjuvant therapy. A consultation with a dietitian with regard to nutritional optimization is recommended.
Evaluation of the patient’s ability to tolerate the surgery: A careful review and optimization of underlying comorbidities (e.g., cardiac, pulmonary, diabetes) and performance
status should be considered in conjunction with other supporting specialties. A subset of high-risk individuals may benefit from preoperative admission to optimize nutrition, electrolyte imbalance, and performance status (e.g., physical therapy) in preparation for their oncologic resection.
Evaluation of response to neoadjuvant therapy: At times, when neoadjuvant therapy is employed to increase rates of R0 resection or spare the GE junction, a repeat posttherapy preoperative EGD provides additional information on tumor response and the proximal extent of the tumor.
Preoperative antibiotics: Patients should be given one dose of first- or second-generation cephalosporin for perioperative antibiotic prophylaxis.
Positioning
In most patients, an upper midline incision provides optimal exposure for the procedure. The chest should be prepped into the operative field for the possibility of needing a thoracotomy for GE junction tumors. A sandbag can be placed under the left chest to facilitate thoracotomy.
TECHNIQUES
STAGING LAPAROSCOPY
Pneumoperitoneum is created by either an open technique or verse needle. A 30-degree scope is inserted at the umbilicus. One to two additional 5-mm ports on either side of the umbilicus are needed for optimal visualization, grasping of tissue, and biopsy of suspicious tissues. A complete survey of the peritoneal cavity is performed, including undersurface of the diaphragm, liver surface, spleen, lining of peritoneal cavity, pelvis, small bowel surface, and omentum for any metastatic disease. If any suspicious lesion is observed, it is biopsied and sent for frozen section. In the setting of biopsy-proven peritoneal disease, gastrectomy should not be performed and nonsurgical treatments should be initiated. However, at times, surgeons are faced with a situation to carefully and selectively consider palliative surgical procedures in the setting of metastatic disease, for example, bleeding or obstructing cancer not palliated by endoscopic means. These decisions need to be individualized based on the performance status of the patient, extent of metastatic burden, the projected survival, and the availability of adjunctive therapy such as endoluminal stenting.
Peritoneal cytology: If the staging laparoscopy is negative for peritoneal spread, the procedure is continued. We perform peritoneal cytology in the patients who do not have gross peritoneal metastasis. For peritoneal cytology, 500 mL of normal saline is instilled into the abdominal cavity and allowed to dwell for 10 to 15 minutes. During this time, the patient’s table is moved from left to right to expose the peritoneum to this fluid. After 10 to 15 minutes, the fluid is aspirated with a suction device, which has a mechanism to trap the fluid. The fluid is then sent to the pathology lab, and in those with positive peritoneal cytology, we favor not proceeding with resection.
When a laparoscopic feeding jejunostomy is to be placed, it is imperative that the surgeon performing the staging laparoscopy and jejunostomy tube placement is cognizant of the need to construct a Roux limb for the restoration of gastrointestinal continuity at the time of definitive surgery. Thus, the loop of jejunum just distal to the ligament of Treitz, which would be used for fashioning the Roux limb, should not be used for the placement of jejunostomy tube.
EXPLORATORY LAPAROTOMY AND MOBILIZATION OF THE LIVER
The abdomen is entered through a midline incision extending from the xiphoid process to just below the umbilicus. A bilateral subcostal incision, approximately 2 cm below the costal margin, also provides good exposure. During entry into the abdomen, the falciform ligament should be preserved as it can be used to buttress the duodenal closure. A careful exploration of the peritoneal cavity is performed to exclude the presence of subradiographic peritoneal or distant metastatic disease. The liver is carefully inspected and palpated for any suspicious nodules.
For better access and visualization of the GE junction, we typically divide the left triangular ligament thus mobilizing the left lobe of the liver. Once mobilized, the left lobe of the liver can be folded on itself thus allowing better visualization of the GE junction and the right crus of the diaphragm.
MOBILIZATION OF THE GREATER CURVATURE OF THE STOMACH
In this step, the attachments of the greater omentum to the transverse colon are divided in an avascular plane. The stomach and the greater omentum are reflected superiorly and the transverse colon is reflected inferiorly. The plane of fusion between the greater omentum and the transverse mesocolon is identified as a faint white line. This plane is entered by incising with electrocautery and, once properly developed, allows access to the lesser sac (FIG 4). This plane of separation is then developed toward both proximal and distal parts of the transverse colon thus completely separating the greater omentum from the transverse colon and mobilizing it with the specimen.
The hepatic flexure of the colon is mobilized by division of the avascular attachment of the hepatic flexure to the retroperitoneum. The separation of the greater omentum from the transverse mesocolon is carried toward the hepatic flexure. This exposes the gastrocolic trunk, which is formed by the confluence of right gastroepiploic vein with middle colic vein and drains into the superior mesenteric vein. The right gastroepiploic vein is divided at its junction with the gastrocolic trunk (FIG 5). Alternatively, the gastrocolic trunk can be divided with a single fire of a vascular stapler. At this stage, the right gastroepiploic artery is divided at its origin from the gastroduodenal artery at the infraduodenal level. We recommend additional time spent when performing this dissection in obese patients given the potential difficulty in identifying this artery. Infrapyloric nodes, located adjacent at the origin of gastroduodenal artery, are mobilized with the specimen (FIG 6).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree