Maria Marinelli
Thyroid and Parathyroid Surgery
Manifestations of thyroid glandular diseases include goiter, benign and malignant nodules, hyperthyroidism, hypothyroidism, and inflammatory conditions. Goiters have been described since 2700 BCE, before the thyroid gland was even identified. During the Italian Renaissance, around 1500, Leonardo da Vinci depicted two glands located on either side of the larynx. In 1543 Vesalius labeled the tissue “laryngeal glands.” However, Thomas Wharton, in a 1656 publication titled Adenograpahia, is credited with replacing the term as the “thyroid gland,” with a Greek origin word (thyreoeides), meaning “shield shaped.” The first documented thyroid surgery occurred in the twelfth century, described by Roger Frugardi. High mortality rates of 40% were the norm until medical advances in anesthesia, antisepsis, and hemostasis were developed during the 1800s. Two notable thyroid surgeons, C.A. Theodore Billroth (1829-1894) and Emil Theodor Kocher (1841-1917), revolutionized the treatment of thyroid diseases. Kocher received the Nobel Prize in 1909 for his advances in thyroid surgery, performing more than 2000 surgeries, with a 4.5% death rate. He captured the title “Father of Thyroid Surgery” for his high rate of surgical achievement in the field of thyroid surgical procedures (Hunter and Jobe, 2010).
Today surgeons continue to perform surgical procedures on the thyroid gland for various underlying causes. Surgery ranges from lobectomy and isthmectomy for papillary microcarcinomas (lesions smaller than 1 cm) to near-total and total thyroidectomies for symptomatic multinodular goiters. Technologic advances have added new approaches to thyroid and parathyroid surgery, such as minimally invasive surgery (MIS) techniques. Emergence of endocrine surgery as a surgical subspecialty that requires advanced postgraduate training has evolved due to the complexity in management of surgical disorders of thyroid, parathyroid, adrenal glands, and neuroendocrine pancreas tumors (Wang, 2011).
Surgical Anatomy
Thyroid Gland
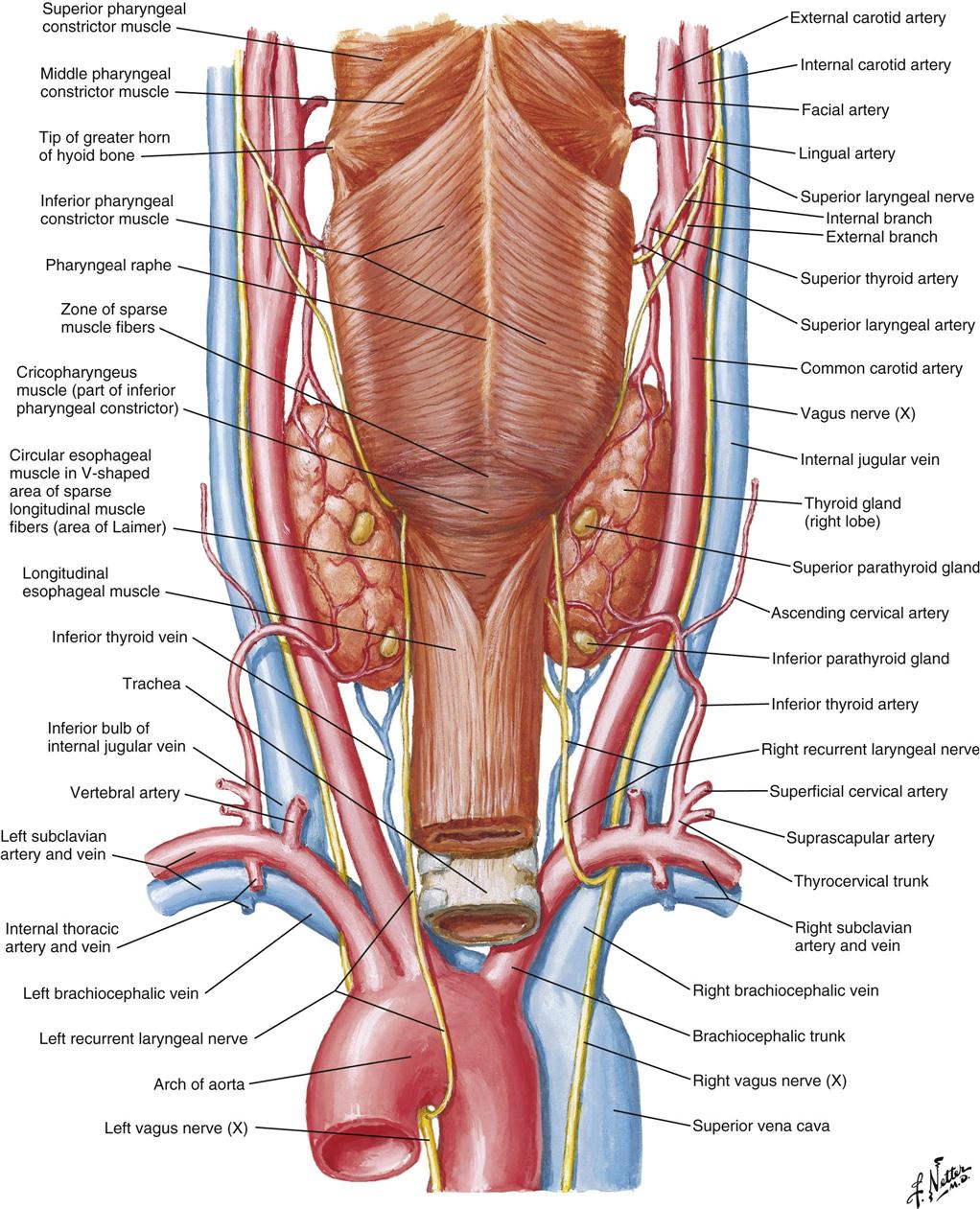
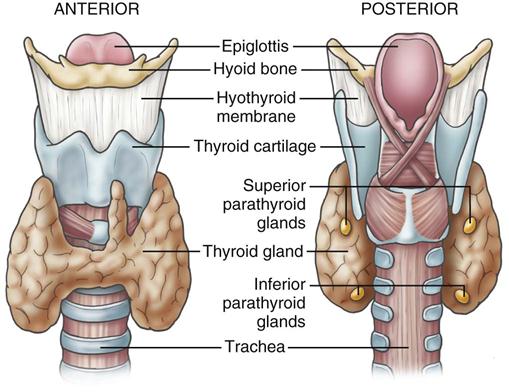
The thyroid gland (Figure 16-1) is a highly vascular organ that lies in the anterior portion of the neck, deep to the paired strap muscles, resting on the midline of the trachea. It is attached to the cricoid and trachea by the ligament of Berry. The inferior border of the thyroid varies and may be located above or below the sternal notch. It consists of right and left lobes united by a middle portion, the isthmus, and it weighs approximately 20 g (Figure 16-2). The isthmus is situated near the base of the neck, between the second and fourth tracheal rings, and the lobes lie beside the larynx, trachea, and esophagus. The pyramidal lobe, a long, thin projection of thyroid tissue protruding cephalad from the isthmus, is found in about 30% of patients at surgery; it is the vestige of the embryonic thyroglossal duct and migrates from the foramen cecum at the base of the tongue.
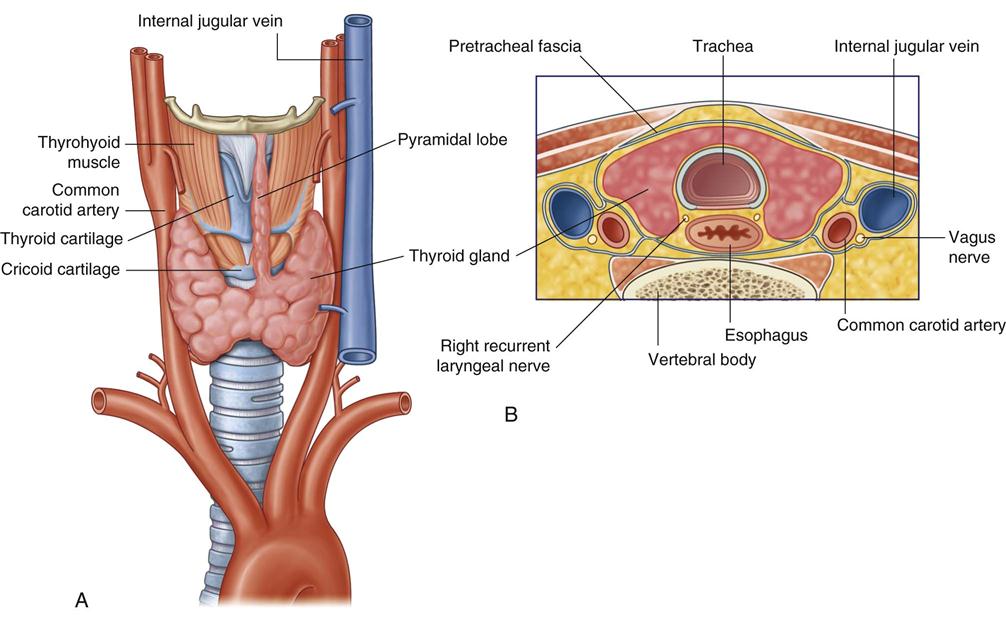
Blood supply to the thyroid is from the superior thyroid artery, which originates from the external carotid artery, and from the inferior thyroid artery, which originates from the thyrocervical trunk of the subclavian artery. The points at which these two arteries enter the thyroid gland are referred to as “poles” of the thyroid and are important anatomic landmarks during surgery. The thyroid gland is drained by three pairs of veins (superior, middle, and inferior thyroid veins). Occasionally a single thyroid artery (thyroidea ima) may arise directly from the arch of the aorta or the innominate artery and rise in front of the trachea to enter the midline of the gland inferiorly.
The recurrent laryngeal nerve, a branch of the vagus nerve, innervates the intrinsic muscles of the larynx. The right recurrent laryngeal nerve loops under the subclavian artery and ascends in an oblique direction lateral to the tracheoesophageal groove. The recurrent laryngeal nerve contains both motor and sensory fibers. The motor component innervates the abductor muscles of the true vocal cords. The sensory component supplies sensation to the larynx below the vocal cords. During surgery, care is taken to identify and protect this nerve. Immediate hoarseness occurs if the nerve is divided on one side. If the recurrent nerve is injured bilaterally, acute paralysis of both vocal cords may obstruct the airway and require emergency tracheotomy. Injury to the external branch of the superior laryngeal nerve, which innervates the cricothyroid muscle, results in difficulty shouting or singing high notes.
The thyroid gland produces three hormones: thyroxine (T4) and triiodothyronine (T3) (together known as the thyroid hormones) and calcitonin. T3 and T4 cannot be synthesized without iodine and regulate energy metabolism and growth and development. Calcitonin inhibits calcium resorption from bone and increases calcium storage in the bone. In addition, calcitonin increases renal excretion of calcium and phosphorus and decreases serum calcium levels. When elevations in serum calcitonin are found in the patient’s blood work, medullary thyroid cancer is suspected. When there is a family history of thyroid cancer, family members are encouraged to seek genetic testing for medullary thyroid carcinoma (Lewis et al, 2011). Thyroid-stimulating hormone (TSH) is synthesized by the anterior pituitary, and stimulates the production and release of thyroid hormones and the uptake of iodine.
Parathyroid Gland
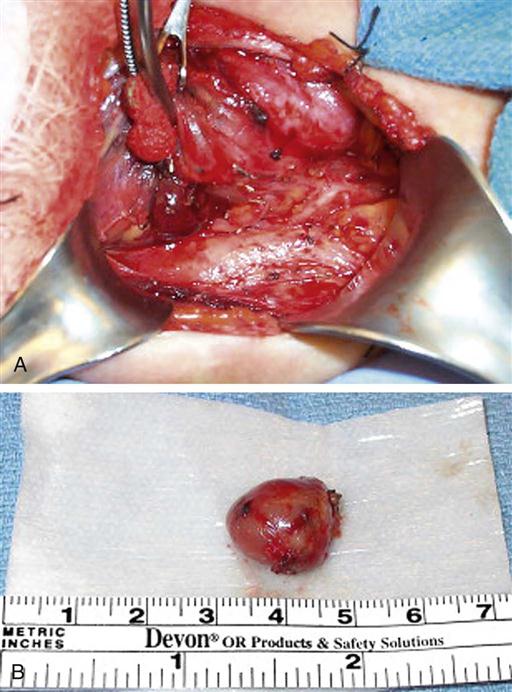
The parathyroid glands are small ovoid masses, usually four in number, situated behind the thyroid lobes. Normal parathyroid glands are generally small, lobular, and yellow, but their color may vary from yellow to mahogany. They are usually found in pairs and are about the size of a lentil bean. It can be challenging to differentiate the gland from a lobule of fat, a thyroid nodule, or lymph node. Thyroid adenomas generally, although not always, tend to be larger, asymmetric, and more red-brown than normal parathyroids (Figure 16-3). The upper pair of glands lies in proximity to the posterior portion of the superior pole of the thyroid; the lower pair usually (about 60% of the time) lies near the posterior lateral aspect of the lower pole of the thyroid. Ectopic parathyroid glands, more common in the inferior parathyroid glands, may be found in the superior mediastinum, especially within the thymus and as low as the pericardium. To allow for interdisciplinary communication between pathologists, radiologists, surgeons, endocrinologists, and anesthesiologists, a classification system is used to determine the most commonly located positions for enlarged parathyroid glands (Harris et al, 2012). Each parathyroid gland measures approximately 3 × 3 × 3 mm and generally weighs less than 50 mg; the upper glands are generally smaller than the lower ones. Both the upper and lower parathyroid glands receive their blood supply from the inferior thyroid artery (Figure 16-4). Because the blood supply to the superior parathyroid stems from that of the supply to the inferior parathyroid, disrupting supply to the inferior parathyroid compromises the supply to the upper parathyroid.
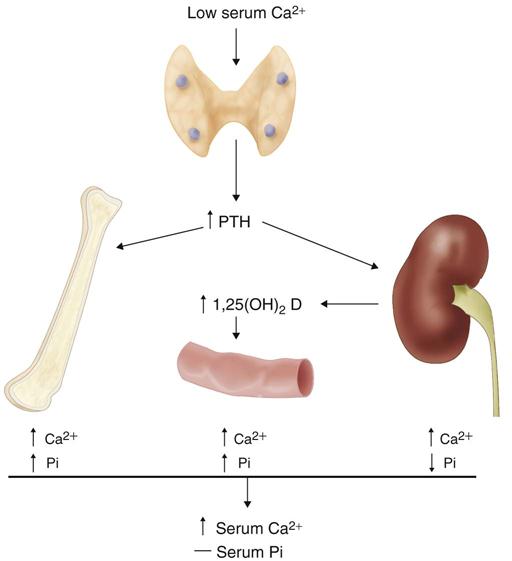
The parathyroid glands secrete parathyroid hormone (PTH), an antagonist to calcitonin. Both PTH and calcitonin work together to maintain calcium homeostasis (Figure 16-5) by increasing calcium removal from storage in bone and increasing absorption of calcium by the intestines. A recent study (Kim, 2012) concludes that an insufficient number of parathyroid glands is the cause of hypocalcemia after total thyroidectomy and node dissection. Therefore, preserving at least one parathyroid gland after a routine thyroidectomy may prevent permanent hypocalcemia.
Perioperative Nursing Considerations
Assessment
Appropriate pharmacologic measures used in treatment of hyperthyroidism include antithyroid drugs, iodine, and β-adrenergic blockers. Medications are considered useful in treating symptoms during the thyrotoxic states and in returning the thyroid hormone levels and metabolism to a normal state. Radiation therapy or surgery is ultimately necessary because the medication regimen is not considered a “cure.” Preoperatively the patient with hyperthyroidism most likely has had appropriate drug therapy that has returned thyroid hormone levels and metabolic state to normal (Lewis et al, 2011). Perioperative nurses assess for any symptoms that relate to accelerated metabolism (Box 16-1). The patient’s baseline vital signs are noted for an abnormally elevated resting pulse rate, elevated systolic blood pressure, and cardiac symptoms, such as palpitations or atrial fibrillation. Studies show that patients with overactive thyroid conditions have an increased risk of various heart problems, particularly atrial fibrillation, that may lead to premature death (Burman and Rodoni, 2012). The perioperative nurse should determine whether anticoagulant and thyroid medications are being taken by the patient. The patient’s cardiac and respiratory rates, muscle strength, elimination patterns, history of weight loss, heat intolerance, and emotional status are reviewed. The patient, especially a young one, may be anxious about the disease state and the success of surgery, and may express concerns about neck surgery and its cosmetic results. Patients who are concerned about body image should have the opportunity to discuss these issues with the perioperative nurse. Vocalists may be especially concerned about whether surgery will adversely affect their voice. The anesthesia provider may choose to use an endotracheal tube with a smaller diameter than usual in order to minimize any trauma to the vocal cords. Skin integrity should be determined; patients with hyperthyroidism may have finely textured skin and edema in the lower extremities, placing them at higher risk for skin breakdown. The most typical complications specific to thyroid surgery, although infrequent, are hypoparathyroidism and injury to the recurrent laryngeal nerve. Preoperative assessment of the patient’s voice tone and quality should therefore be noted.
Large thyroid nodules may cause tracheal shift and present a challenge to the anesthesia provider for intubation and airway management. The size of thyroid enlargement should be noted preoperatively and steps taken to prepare the operating room (OR) in anticipation of a difficult intubation. The “difficult airway” anesthesia cart should be in the room along with, preferably, two anesthesia providers and the circulating nurse standing by to assist with induction. The anesthesia provider will not extubate a patient who has been difficult to intubate until the anesthestic gases have dissipated and level of consciousness is such that the patient can protect his or her own airway and maintain oxygen saturation with spontaneous breathing. If there is concern about postoperative edema and ensuing airway obstruction from a difficult induction, the anesthesia provider may administer steroids intraoperatively and choose to leave the tube in postoperatively.
Grossly enlarged thyroids are also more difficult to excise, and intraoperative bleeding is more common and profuse. Finger dissection may be unavoidable and small vessels that retract and contract may rupture during emergence or postoperatively, leading to hemorrhage. Hemorrhage may cause tracheal compression and airway compromise. Generally, patients who have larger glands excised will require a drain for postoperative wound drainage (Byrne, 2013). They must be closely monitored during emergence from anesthesia and postoperatively; excessive swelling at the operative site, with or without poor air exchange, must be treated as a potentially life-threatening emergency. The instrument table should be kept sterile until the patient is extubated and has left the OR. A tracheotomy tray with tubes should be readily available should an emergency tracheotomy be necessary.
In addition to clinical signs and symptoms, results of diagnostic tests should be reviewed (Evidence for Practice). Tests performed most commonly before thyroid surgery include measurements of TSH, T3, and T4 levels; radioisotope or ultrasonic scans; and an electrocardiogram (ECG).
Thyroid Assessment.
In addition to palpation of the thyroid gland for size, contour, consistency, lymph nodes, fixation, tenderness, and bruits, ultrasound is often the first diagnostic test performed (Lewis et al, 2011). Computed tomography (CT) and magnetic resonance imaging (MRI) scans are used to assess a thyroid with suspected malignancy for encroachment of the tumor outside the thyroid capsule into the adjacent tissue of the neck. Scans are the most effective means to evaluate local invasion of surrounding tissue.
Ultrasound and autopsy studies show more than 50% of healthy adults have thyroid nodules, yet only 3% to 5% are found to be palpable. Although most nodules are benign, 7% to 29% are cancerous. There are an estimated 60,000 new cases of thyroid cancer each year, with a 3:1 female-to-male ratio (ACS, 2013). The surveillance program of the National Cancer Institute estimates the prevalence of thyroid cancer is less than 1% of the population (Harris et al, 2012). The lifetime risk of developing thyroid cancer is 1 in 97 (Howlader et al, 2013). From 2005 to 2009, the median age at diagnosis for cancer of the thyroid was 50 years. Approximately 1.8% were diagnosed younger than age 20; 15.5% between ages 20 and 34; 20.4% between ages 35 and 44; 24% between 45 and 54; 19% between 55 and 64; 11% between 65 and 74; 5.9% between 75 and 84; and 1.4% at 85 years and older. Thyroid cancer is poised to become one of the most common cancer diagnoses in living cancer patients (Grebe, 2009).
Preoperative Testing
Thyroid Isotope Scan (Thyroid Scintigraphy).
A thyroid nodule, the most common endocrine problem in the United States, is any abnormal growth of thyroid cells. Most nodules are discovered during routine physical examination, because they rarely are symptomatic. Imaging of the normal thyroid with radionuclide agents shows normal size, shape, position, and function of the thyroid, with no areas of decreased or increased uptake. Nodules that are hot demonstrate increased uptake of radionuclide agent; this may indicate benign adenoma or localized toxic goiter. Cold nodules are hypofunctioning or nonfunctioning and may indicate cyst, nonfunctioning adenoma or goiter, lymphoma, localized area of thyroiditis, or carcinoma. Two commonly used agents in thyroid imaging are radioactive iodine and technetium 99m-pertechnetate (99mTcO4−).
Ultrasonic Scan.
Ultrasonic scans are the treatment of choice to evaluate thyroid nodules and are useful to determine the size and number of nodules within the thyroid as well as to monitor size progression during follow-up. Involvement of the vessels in the neck or mediastinum, although rare, can occur. It is critical that the surgeon and operative team know this before surgery. Radiologic evaluation frequently dictates the extent of the surgery. Several new ultrasonic techniques have been applied to the thyroid. Three-dimensional (3D) ultrasound has proven to be more accurate. Tissue harmonic imaging (THI) uses the first harmonic of the transmitted frequency for image formation and results in images with less “noise.” These techniques improve image clarity but do not improve lesion characterization.
Ultrasonography also helps facilitate accurate needle placement and sampling of the target nodule during fine-needle aspiration (FNA).
Fine-Needle Aspiration.
When a tissue sample for pathologic examination is required, FNA of a palpable nodule is considered one of the most effective methods for identifying malignancy. FNA has high accuracy and low risk, and is cost-effective. A suspicious nodule usually implies a follicular neoplasm, which can be benign or malignant, and requires removal and evaluation by the histology department. The procedure is usually performed under ultrasound guidance, but may be performed in the surgeon’s office. Several samples are usually taken from various parts of the nodule, and are rated on a 1 to 6 scale ranging from benign to clearly malignant. When metastatic disease is found on preoperative imaging, an altered operative and disease management plan will be established for the patient. FNA is not suggested for diagnosing parathyroid carcinoma because of the potential for seeding or spreading of the malignant parathyroid cells (Harris et al, 2012). Patient preparation for FNA should include the following information:
• Local anesthesia may or may not be used.
• Once the prick of the needle is felt, no talking, swallowing, or moving is allowed.
FNA may be an emotionally stressful procedure for the patient. Vials of ammonia should be kept in the room in case the patient feels faint. Lowering the head (using Trendelenburg position or sitting with the head down) is also important to treat vasovagal syncope. Postprocedure education should include (1) the necessity of refraining from using aspirin or aspirin-containing medications or nonsteroidal antiinflammatory drugs (NSAIDs) for the next 24 hours, and (2) the expectation of seeing a half-dollar–size bruise at the FNA site (Patient-Centered Care). There are no restrictions on food or activity.
Preoperative Patient Education.
Preoperative patient education for partial or total thyroidectomy includes an explanation of perioperative events, discussion of the incision site and type of dressing to be used, and explanation of any drains, if anticipated. Pain assessment and pain management therapy are reviewed. Patients are advised that there are various ways of protecting their eyesight during surgery, and if ointment is used, they can expect temporary blurred vision when they first open their eyes. If intermittent pneumatic compression devices are to be used for deep vein thrombosis (DVT) prophylaxis, their use and sensation is explained. The patient is encouraged to flex and extend the neck slowly early in the postoperative period. As mentioned, a baseline description of vocal cord function is noted preoperatively. (For further discussion of patient education, see Patient and Family Education and Discharge Planning on page 547.)
Parathyroid Assessment.
Elevation of serum PTH level in association with hypercalcemia is diagnostic of hyperparathyroidism in most cases. Normal PTH levels are between 15 and 65 pg/mL; also diagnostic is a serum calcium level greater than 1 mg/dL above normal (8.5 to 10 mg/dL) and a bone density T-score of less than –2.5 at any site (Yeh et al, 2012). Causes of primary hyperparathyroidism are single adenoma (80% to 85%), four-gland hyperplasia (10% to 15%), and rarely parathyroid carcinoma (less than 1%).
Nuclear Medicine.
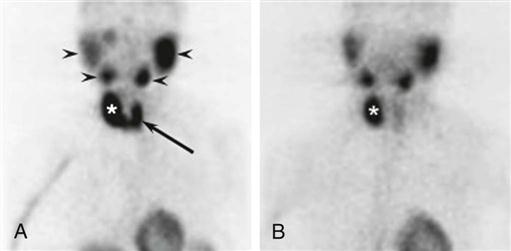
Preoperative localization studies with a parathyroid sestamibi scan (Figure 16-6) or high-resolution ultrasonography can identify parathyroid adenomas with high sensitivity (70% to 80%). Sensitivity decreases significantly for hyperplasia (less than 50%).
Improvements in anatomic detail may be achieved by using the single-photon emission computed tomography/CT (SPECT/CT). This system uses software that depicts 6 to 16 slices of tissue and produces 3D images that help differentiate the parathyroid tissue from lymphadenopathy and thyroid pathologies (Harris et al, 2012).
Four-Dimensional CT.
This uses a multiphase multidetector CT that provides rapid volumetric acquisition and in-plane spatial resolution of 1 mm or better, which provides a roadmap for the operating surgeon. There is more precise visualization of the parathyroid glands, and detailed anatomic information provided with this CT scan (Harris et al, 2012).
Hyperparathyroidism causes an increase in the level of serum calcium and a decrease in the level of serum phosphate. Nursing diagnoses and plans of care are based on these imbalances and the severity of associated symptoms. Some patients are asymptomatic, whereas others have symptoms that manifest themselves as disturbances in the central nervous system or cardiovascular, renal, gastrointestinal, or musculoskeletal system (Box 16-2).
Assessment includes determining whether the patient is apathetic or emotionally irritable; whether there is muscle weakness and atrophy, back or joint pain, nausea, vomiting, constipation, peptic ulcer disease, or cardiac dysrhythmia; and whether there is renal damage, stones, or disease. If any of these are present, the plan of care is adjusted. Otherwise, perioperative patient education and nursing management of the patient undergoing parathyroidectomy are essentially the same as those for thyroidectomy. In the early postoperative period for both thyroidectomy and parathyroidectomy, the patient is closely observed for any signs of hypocalcemia. The serum calcium level reaches its lowest level in 48 to 72 hours after surgery and returns to normal within the following 2 to 3 days. Symptoms include numbness and tingling of extremities and around the lips. Hyperactive tendon reflexes and a positive Chvostek sign (tapping on the facial nerve elicits contraction of facial muscles) can be demonstrated on physical examination. Tetany may develop and is exhibited by carpopedal spasms, tonic-clonic convulsions, and laryngeal stridor, which can be fatal.
Nursing Diagnosis
Nursing diagnoses related to the care of patients undergoing thyroid and parathyroid surgery might include the following:
Outcome Identification
Outcomes identified for the selected nursing diagnoses may be stated as follows:
Planning
The perioperative nurse and surgical technologist should know the OR team members who will participate in the procedure as well as the roles each team member will assume (Haynes et al, 2009). Careful planning, assessment, and tailoring the plan of care to the surgery and specific patient needs reduce inherent risks. The perioperative nurse should consider a patient who is not at optimal weight at high risk for pressure ulcer development and plan on padding pressure areas to prevent skin and tissue damage. Intraoperatively, warm saline should be provided for irrigation and a forced-air warming device used. Patients with hypothyroidism are at increased risk of becoming hypothermic (Hegarty et al, 2009).
A rapidly progressive and potentially fatal complication for patients with hyperthyroidism is thyroid storm (thyrotoxic crisis) (Copstead and Banasik, 2010). The perioperative staff must recognize and differentiate between uncomplicated thyrotoxicosis and thyroid storm (Box 16-3) and be prepared to act quickly. Thyroid storm can occur in patients whose hyperthyroidism has been partially controlled or has been left untreated. Thyrotoxic crisis can be precipitated by a stressful event, such as surgery. By planning a quiet, calm atmosphere and helping the patient relax, the perioperative nurse can reduce the risk of thyroid storm. Collaborating with the surgical and anesthesia team, the perioperative nurse plans for appropriate interventions to assist in reducing body temperature and heart rate, provides oxygen and intravenous solutions, and administers medications as prescribed in the event thyrotoxic crisis occurs (Surgical Pharmacology).
Surgical Pharmacology
Thyroid Storm

IM, Intramuscular; IV, intravenous; NG, nasogastric; PO, orally.
Modified from Copstead LE, Banasik J: Pathophysiology, ed 4, St Louis, 2010, Saunders; Crawford A, Harris H: Thyroid imbalances: dealing with disorderly conduct, Nursing 2012 42(11):45–50, 2012; Czako P, Meyers A: Thyrotoxic storm following thyroidectomy, available at emedicine.medscape.com/article850924. Accessed May 27, 2013; Simmons S: Detecting thyroid disease, Nursing 2010 40(7):22–29, 2010.