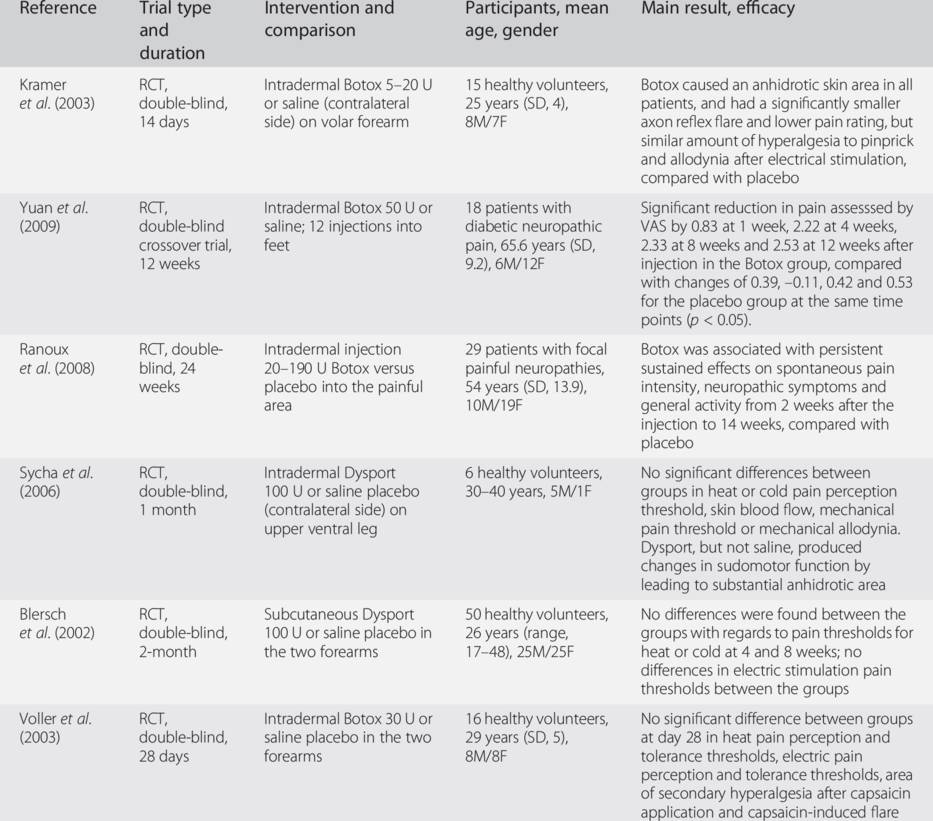
Botox, onabotulinumtoxinA; Dysport, abobotulinumtoxinA; SD, standard deviation; RCT, randomized controlled trial; M, male; F, female; VAS, visual analog scale.
Refractory osteoarticular joint pain
Table 27.2 summarizes the efficacy data from two non-randomized studies (Mahowald et al., 2006; Singh et al., 2009a) followed by data from randomized controlled trials (RCTs) in refractory shoulder joint pain (one study) and refractory knee joint pain (two randomized studies) caused by osteoarthritis or inflammatory arthritis (Singh et al., 2009b; Mahowald et al., 2009; Boon et al., 2010). These studies have all used onabotulinumtoxinA (Botox). In one RCT comparing a single intra-articular injection of 100 U onabotulinumtoxinA with placebo for shoulder pain in osteoarthritis onabotulinumtoxinA was superior to placebo in pain reduction and quality of life improvement (Singh et al., 2009b). In a RCT comparing a single intra-articular injection of two doses (100 or 200 U) of onabotulinumtoxinA with intra-articular methylprednisolone acetate for knee osteoarthritis pain, onabotulinumtoxinA was as effective as the corticosteroid in improving pain, function and quality of life (Boon et al., 2010). In a RCT comparing a single intra-articular injection of 100 U onabotulinumtoxinA with intra-articular placebo, significantly greater reduction in pain was seen in the group with severe pain (≥7 on 0–10 scale) with onabotulinumtoxinA compared with placebo. No deaths, anaphylactic reaction or septic arthritis were reported in any group in any studies and strength testing did not reveal any significant changes at any time point in treatment groups. None of the common adverse events were significantly different between groups, in the three RCTs (Mahowald et al., 2009; Singh et al., 2009b; Boon et al., 2010). These three small trials were powered for efficacy but not safety outcomes.
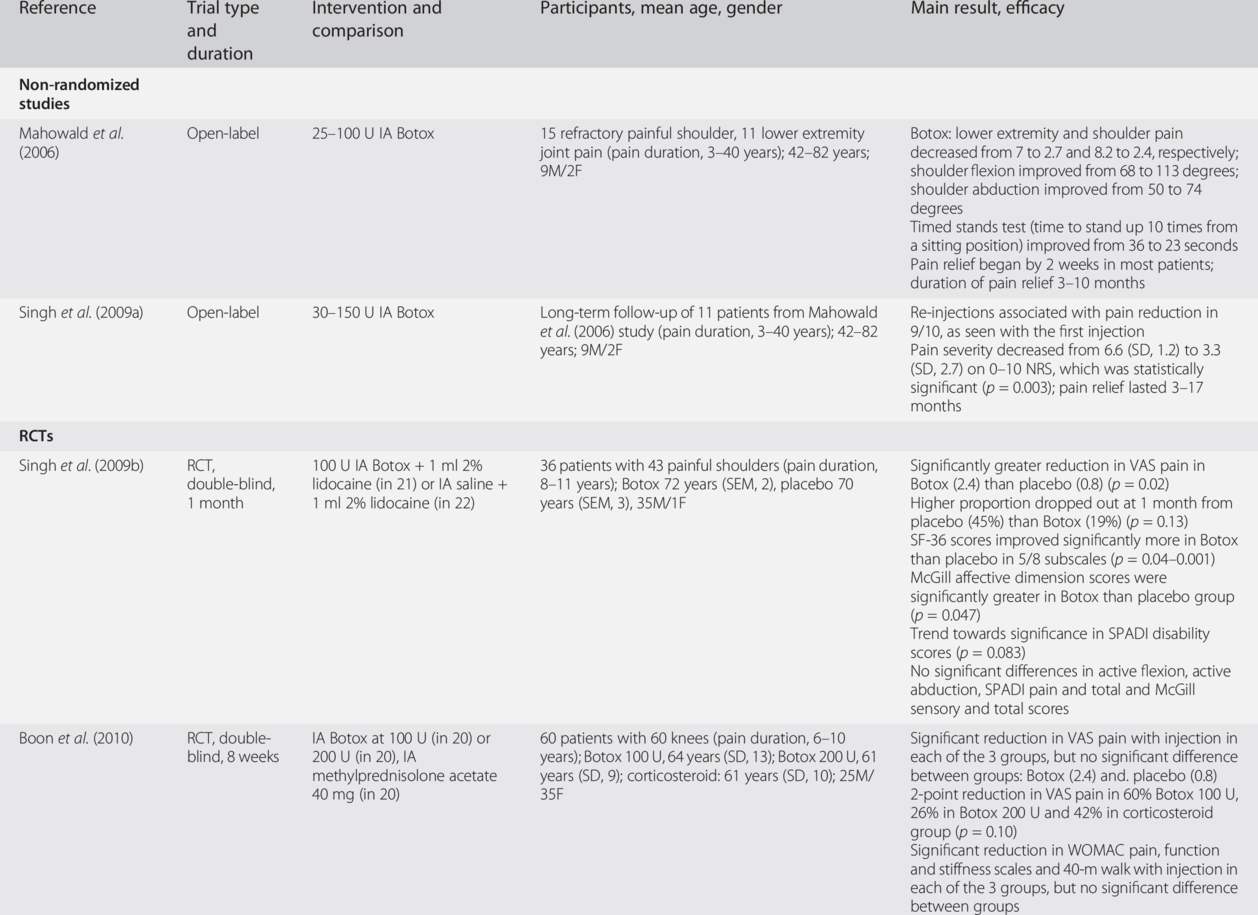

Botox, onabotulinumtoxinA; RCT, randomized controlled trial; M. male’ F. female; VAS, visual analog scale; SPADI, Shoulder Pain and Disability Index; WOMAC, Western Ontario McMaster Osteoarthritis Index; NRS, numeric rating scale; SD, standard deviation; SEM, standard error of mean; IA, intra-articular.
In summary, three RCTs and three non-randomized studies support the efficacy of a single intra-articular injection of BoNT in patients with refractory joint pain. However, larger dose-ranging studies with safety assessments are needed.
Knee injection can be performed by diluting 100 U onabotulinumtoxinA into 1 or 2 ml of normal saline and injecting it into the knee joint using a 21- or 22-gauge needle and any of the approaches (medial, lateral, superior or inferior) according to personal preference. Other formulations of BoNT can presumably be used, but there is less experience with them. The shoulder can be similarly injected with a 21- or 22-gauge needle using posterior, anterior or lateral approach to the glenohumeral joint, according to personal preference. Although not recommended by the manufacturer, some clinicians prefer using 1% lidocaine to dilute the BoNT instead of normal saline to confirm the correct delivery of the medication by improvement in pain 5 minutes after the injection (Fig. 27.1).
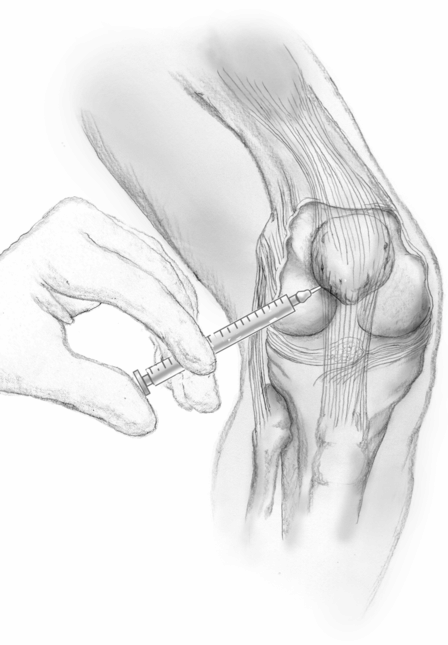
Fig. 27.1 Technique for intra-articular injection of the knee joint using the medial approach.
Tennis elbow
The efficacy of local injection of BoNT into the extensor tendons inserting on the external epicondyle for improving pain was examined in an open-label case series of 14 patients with “treatment-resistant” tennis elbow with follow-up at 6–8 months (Morre et al., 1997). This was confirmed in a 12-week RCT of 60 patients comparing BoNT with placebo (Wong et al., 2005) (Table 27.3). Four patients in the abobotulinumtoxinA (Dysport) group had paresis of the fingers at 4 weeks (in one patient persisting to week 12) compared with none in the placebo group, but grip strength was similar in both groups at the two time points (Wong et al., 2005). Another RCT in 40 patients with tennis elbow found that a single injection of 40 U onabotulinumtoxinA seemed to be as effective as surgical release for improvement of pain, but led to a better range of motion at 3 and 6 months (Keizer et al., 2002) (Table 27.3). A third RCT compared a single injection of 50 U onabotulinumtoxinA into the extensor tendons with placebo (Hayton et al., 2005). There were no statistically significant differences by treatment in pain (difference of 1.1 between groups; p = 0.54), grip strength (difference of 0.57 kg between groups; p = 0.90), or quality of life; 12 of the 18 patients in the onabotulinumtoxinA group had a transient extensor lag of the long finger at 1 week that disappeared 3 months after the injection (Hayton et al., 28).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


