normal in tumors. Therefore, our approach in evaluating a palpable thyroid mass is to begin with fine-needle aspiration biopsy, which we found to be a safe procedure as have many others (Castro and Gharib, 2000; Werga et al, 2000; Mazzaferri, 1993; Gagneten et al, 1987; Rojeski and Gharib, 1985; Löwhagen and Willems, 1981; Wang et al, 1976; Löwhagen and Sprenger, 1974). Using this approach, we have performed approximately 10,000 fine-needle aspiration biopsies of the thyroid, most guided by palpation and the others under ultrasound guidance.
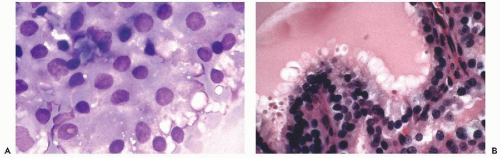
pure colloid may be consistent with the diagnosis of benign colloid goiter and a pure population of macrophages may be diagnostic of a thyroid cyst or pseudocyst in a patient with past history of hemorrhage. In neither instance is the presence of epithelial cells required for diagnosis. Clearly, the issue depends on the type of lesion (cystic or solid) and the skill of the performer. It has been our experience that the latter is paramount.
Cysts
Goiters
Colloid goiter
Thyroiditis
Acute
Subacute (deQuervain’s)
Lymphocytic (Hashimoto’s disease)
Riedel’s Struma (fibrosing thyroiditis)
Tumors
Follicular tumors
Follicular adenomas
Follicular carcinoma
Hürthle cell tumors
Hürthle cell adenoma
Hürthle cell carcinoma
Other carcinomas
Papillary and its variants
Medullary
Anaplastic (large- and small-cell types)
Malignant lymphomas
Rare malignant tumors
Metastatic tumors
Acute thyroiditis
Cyst with acute hemorrhage
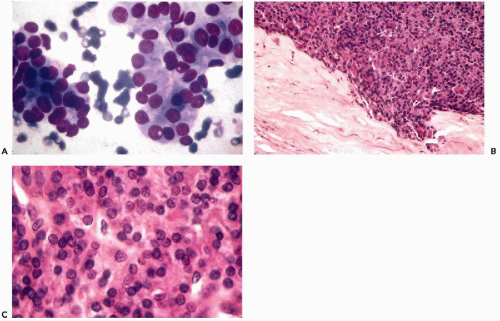
Subacute or De Quervain’s thyroiditis
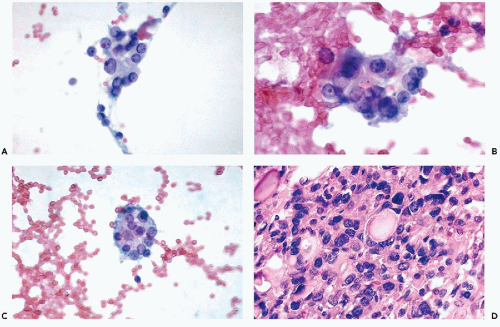
Infarcted Hürthle cell tumor (rare)
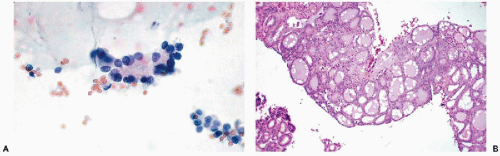
Hashimoto’s thyroiditis
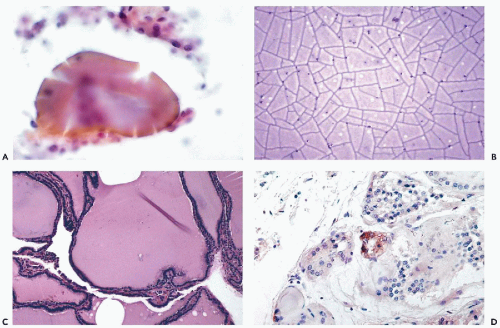
duct are located in the midline, usually above the thyroid, and are lined by either cylindrical or squamous epithelium (see below). A case of an intrathyroid lymphoepithelial cyst of probable branchial origin was described by Apel et al (1994). It must be stressed that some malignant tumors, particularly papillary carcinomas, are often partly cystic.
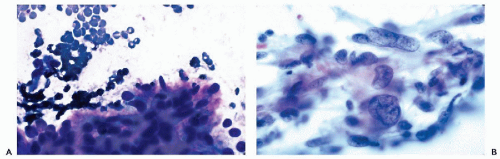
thyroiditis to chronic inflammatory processes. The term also includes a number of transient disorders such as postpartum thyroiditis and drug-induced thyroiditis (recent review in Pearce et al, 2003). Only the common forms of thyroiditis should be sampled by FNA and they are discussed below.
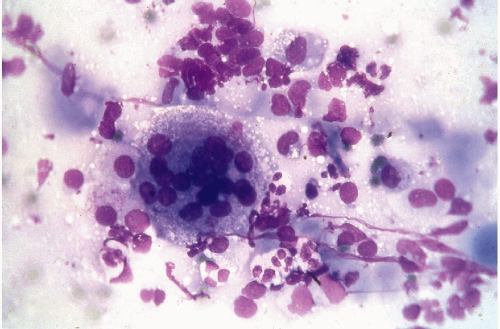 Figure 30-6 Subacute thyroiditis. The smear contains a large multinucleated giant cell surrounded by debris. (Diff-Quik stain.) |
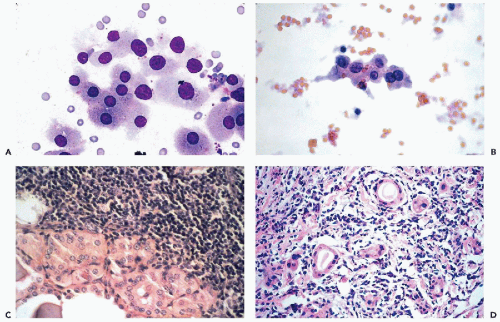
may occur in Hashimoto’s thyroiditis. There is a misperception that malignant lymphoma is the most common tumor seen in Hashimoto’s thyroiditis. In fact, from 5% to 10% of patients with this disease will develop papillary carcinoma, and less than 1% develop malignant lymphoma (Carson et al, 1996).
Further, the presence of abundant lymphocytes is very uncommon in Hürthle cell tumors.
TABLE 30-1 KEY MORPHOLOGIC FEATURES OF DIFFERENTIAL DIAGNOSIS AMONG COMMON THYROID TUMORS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
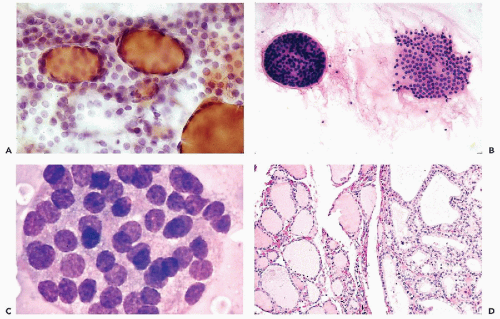
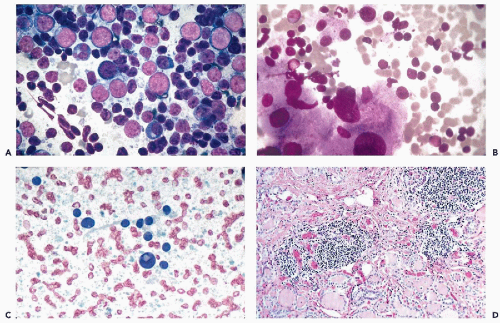
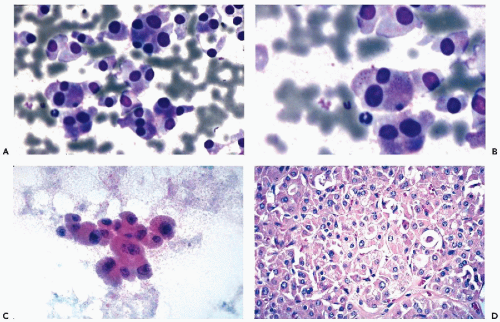
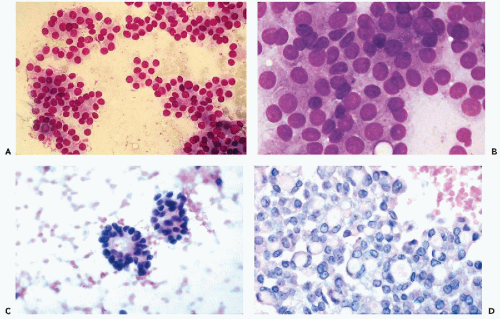 Figure 30-10 Follicular neoplasms. A,B. Flat spherical clusters of follicular cells stained with MGG. Note the absence of colloid and the somewhat enlarged nuclei, when compared with normal acini shown in Figure 30-2. C. One acinar and one solid cluster of follicular cells (Diff-Quik). D. Histologic section of a follicular adenoma, corresponding to C. Note small follicles containing scanty, pale colloid. |
visible. Small, indistinct intranuclear clear inclusions may occur and do not necessarily indicate a malignant tumor (see Fig. 30-10B). The papillary configuration of some of the colloid-free clusters may be reminiscent of papillary carcinoma. However, the clusters in papillary cancer are often multilayered and complex and show specific nuclear abnormalities that are not evident in follicular tumors (see below). Aspiration smears from follicular carcinomas are also highly cellular with little or no colloid. The cells occur primarily in clusters and are often arranged in follicle-like structures. Thus, they may closely resemble the cytologic presentation of follicular adenoma. In well-differentiated follicular carcinoma, cellular atypia may be minimal, and the general impression from the smear may suggest a benign lesion, rather than a carcinoma (Fig. 30-11). Therefore, in such cases, the cytologic diagnosis should be “follicular neoplasm or tumor,” clearly indicating that surgical excision and histologic examination is mandatory for a reliable differential diagnosis between follicular adenoma and a well-differentiated carcinoma.
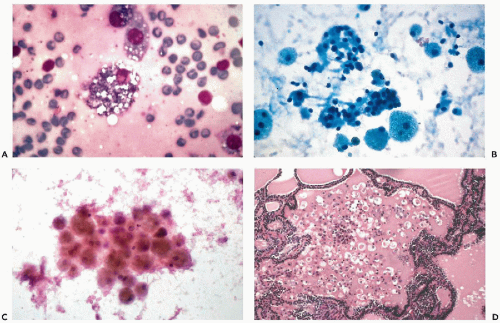
and do not recur or metastasize after careful surgical removal. A papillary variant of Hürthle cell tumor and a variant of papillary carcinoma composed of Hürthle cells with lymphoid stroma (Warthin’s-like tumor) are discussed below with papillary carcinomas. Fewer than 10% of the Hürthle cell tumors invade the capsule, blood vessels, and adjacent organs and, therefore, must be considered malignant (Fig. 30-15D). Because of their unpredictable behavior, surgical removal of these tumors is the treatment of choice (Nguyen et al, 1999). Infarction of these tumors is an infrequent, but known, complication of FNA (Kini, 1996; Pinto and Mandreker, 1996).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree