Effusions in the Absence of Cancer
ANATOMY AND HISTOLOGY OF THE PLEURAL, PERITONEAL, AND PERICARDIAL CAVITIES
The organs contained within the body cavities are surrounded by a thin membrane, sometimes referred to as serous membrane, or serosa. The leaf of the membrane surrounding the organs is known as the visceral layer. The visceral layer extends to the outer walls of the body cavity where it forms the parietal layer. The visceral and the parietal layers are in continuity with each other and thus form a self-contained, closed cavity that is not in contact with the outside world.
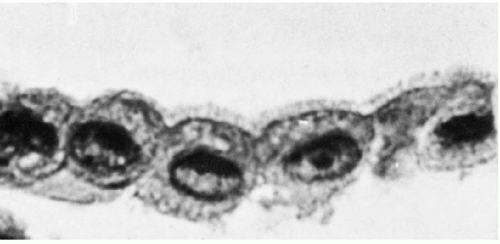
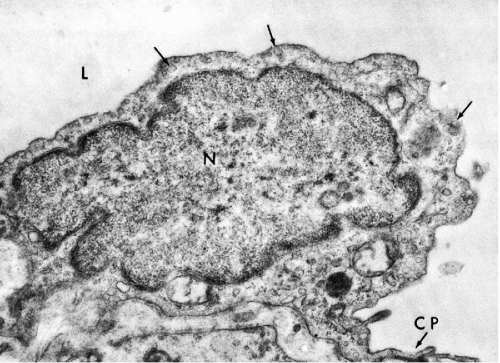
The three body cavities (the pleura, peritoneum, and pericardium) have a common embryologic origin in the mesenchymal embryonal layer. The pleura encloses the lungs; the peritoneum, the intestinal tract; and the pericardium, the heart (Fig. 25-1). The serous membranes are lined by the mesothelium composed of a single layer of flat cells, supported by connective tissue, and an appropriate vascular and nervous apparatus (Fig. 25-2). Under favorable circumstances of perfect fixation and gentle processing, a brush border may be observed on the luminal surface of the mesothelial cells (Fig. 25-3).
In the absence of disease, the parietal and the visceral layers of the mesothelium are separated by a thin layer of lubricating fluid that facilitates the movements of the two membranes against each other. The flow of the lubricating fluid is regulated by mesothelial cells.
Transmission electron microscopy of the mesothelium reveals a continuous single layer of cells bound to each other by demosomes and gap junctions, with surface microvilli extending toward the body cavity (Policard et al, 1955; Odor, 1956; Felix, 1961; Cotran and Karnovsky, 1968; Efrati and Nir, 1976). Pinocytotic vesicles are numerous at both poles of the mesothelial cells (Fig. 25-4). Scanning electron microscopy confirmed that the mesothelial cells lining the surface are provided with short microvilli of equal length. The microvilli of normal mesothelial cells are too small to be visible in light microscopy. The presence of longer, visible microvilli on the surfaces of mesothelial cells usually indicates a disease process, as will be discussed in Chapter 26.
ACCUMULATION OF FLUIDS (EFFUSIONS) IN BODY CAVITIES
Under pathologic circumstances, the two leaflets of the serous membrane may be separated from each other either
because of the presence of air or fluids within the body cavity. The presence of air constitutes, respectively, a pneumothorax, a pneumoperitoneum, or a pneumopericardium.
because of the presence of air or fluids within the body cavity. The presence of air constitutes, respectively, a pneumothorax, a pneumoperitoneum, or a pneumopericardium.
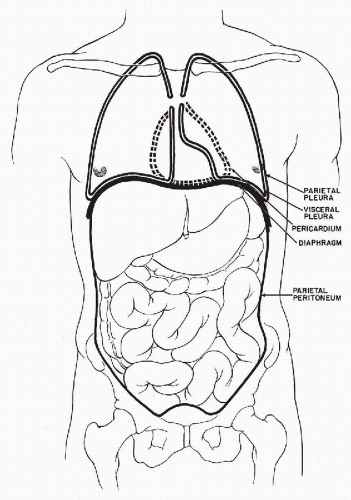 Figure 25-1 Schematic representation of the three body cavities. The visceral layer of the peritoneum is identical with the serosal lining of the intestinal tract. |
From the point of view of diagnostic cytology, the accumulation of excess fluids in the body cavities is of capital significance. The mere presence of a fluid in any of the body cavities indicates a pathologic process. The presence of blood in one of the body cavities, usually caused by trauma or rupture of a viscus, results in a hemothorax, hemoperitoneum, or hemopericardium. These conditions are irrelevant in the context of this book.
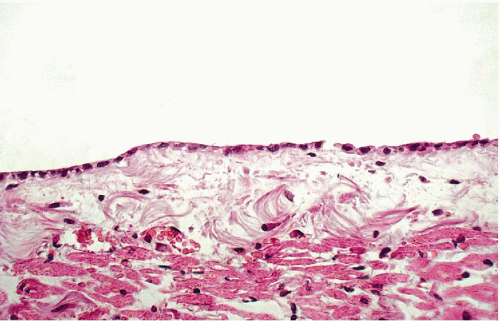 Figure 25-2 Normal mesothelial lining of the pericardium. There is a single layer of flat cells on the surface of supporting connective tissue. |
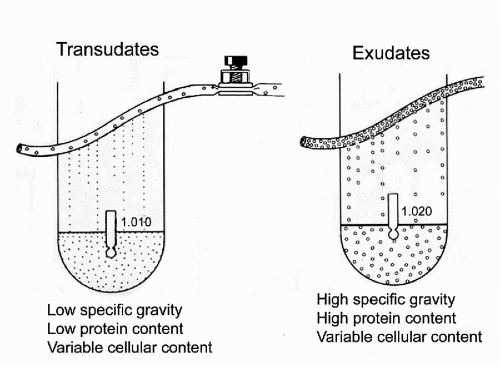
The presence of fluid other than blood constitutes an effusion, which in the abdomen is called ascites. Although the usual purpose of cytologic investigation in such cases is to determine the presence or absence of tumors cells, many other conditions can also be identified. The fluids or effusions may be classified as transudates or exudates (Fig. 25-5).
Transudates
The transudates are clear, straw-colored fluids, characterized by a low specific gravity, often below 1.010, and low protein
content (usually below 3 g/100 ml). The accumulation of transudates within body cavities is caused by filtration of blood serum across the physically intact vascular wall either by reduced intravascular osmotic pressure, as in hypoproteinemia, or by increased filtration pressure, as in heart failure.
content (usually below 3 g/100 ml). The accumulation of transudates within body cavities is caused by filtration of blood serum across the physically intact vascular wall either by reduced intravascular osmotic pressure, as in hypoproteinemia, or by increased filtration pressure, as in heart failure.
The cellular components of transudates are scanty and are limited to a few mesothelial cells and leukocytes. When the cytologic components of an effusion are more complex, the effusion is, in all likelihood, an exudate.
Exudates
The exudates result from an accumulation of fluid within the body cavities, associated with damage to the walls of the capillaries. The exudates are cloudy or opaque fluids of various colors. The presence of blood-tinged, reddish fluid is an important diagnostic sign that should be recorded because it may indicate the presence of a primary or metastatic tumor or tuberculosis.
The exudates are characterized by a relatively high protein content (usually above 3 g/100 ml) and, therefore, a high specific gravity (usually above 1.015). The exudates are rich in fibrin and may coagulate on standing and usually contain a variable, but often significant, population of cells that are the target of cytologic investigations. There are several causes of exudates:
Inflammatory conditions that are usually, but not always, caused by infectious processes in the organ enclosed by the serous membrane
Tumors that may be primary or metastatic
Miscellaneous causes, discussed below
Storey et al (1976) stated that cytologic examination and determination of protein levels were the most useful methods of assessment of pleural effusions: in the great majority of cancer patients, the effusions had protein levels of 3 g/100 ml or more. Protein values below 3 g/100 ml occurred mainly in patients with congestive heart failure, the principal cause of chronic effusions in the absence of cancer.
A very rare form of abdominal effusion is chylous effusion caused by a rupture or obstruction of thoracic duct that carries whitish liquid lymph from abdominal lymph nodes to the heart.
METHODS OF INVESTIGATION OF EFFUSIONS
The customary methods of investigation of effusions are cytologic examination of the fluid and a biopsy of the pleura, pericardium, or peritoneum, sometimes supplemented by immunocytologic, biochemical, bacteriologic or cytogenetic investigation. Light (2002) discussed in detail the clinical and biochemical indications for thoracentesis and cytologic examination of pleural effusions.
The technical details of processing fluid samples are discussed in Chapter 44. The cytologic examination is usually performed on smears of centrifuged sediment that may be prepared by manual methods or by some of the newer methods of processing of liquid samples. The smears may be either fixed immediately and stained by the Papanicolaou method or, as some observers prefer, air-dried, fixed in methanol, and stained by using one of the modifications of the May-Grünwald-Giemsa (MGG) stain.
It is strongly advised to process the remainder of the specimen as a cell block which can provide structural information and can be used for special stains or immunostaining. In a recent extensive review of the subject of immunocytochemistry in effusions, Gong et al (2003) noted that samples processed by the ThinPrep (Cytyc Corporation, Boxborough, MA) method were equivalent to cell blocks, except for markers of nuclear proliferation. Thus, in our judgment, there is no reason to abandon cell blocks as the least expensive and satisfactory target for ancillary studies.
A number of biochemical methods has been used in the evaluation of effusions. Thus, the level of lactic dehydrogenase and other enzymes, several antigens such as human chorionic gonadotrophin (hCG), carcinoembryonic antigen (CEA), and CA-125 may be measured. The value of these procedures in diagnosis or in monitoring the effects of treatment is being debated. They sometimes supplement, but never replace, the cytologic examination.
Many immunocytologic procedures may serve to identify and classify cancer cells in effusions (Coleman and Ormerod, 1984; Johnston, 1987; Bedrossian, 1994). The technical aspects of these procedures are discussed in Chapter 45. The subtyping of lymphocytes in effusions, either by immunochemistry or flow cytometry, is sometimes helpful in the assessment of lymphocyte populations in various conditions but is indispensable in subclassification of malignant lymphomas (see Chaps. 26 and 31 for further discussion).
Cytogenetic analysis of cells in fluids may yield important data on the nature of the effusion, particularly in difficult diagnostic situations. There are two modes of cytogenetic analysis. The older method calls for examination and analysis of chromosomes in metaphases in cultured cells. Newer approaches utilize specific DNA probes to chromosomes, individual genes or targeted translocations by fluorescent in situ hybridization (FISH) method, applied to interphase nuclei.
DNA ploidy analysis and molecular analysis of tumor cells by methods such as Southern blotting (discussed in Chap. 3) may also be applied to select populations of cells in effusions. The value of these procedures and of tissue biopsies in the diagnosis of effusions is discussed in this chapter and in Chapter 26.
CELL POPULATIONS IN BENIGN EFFUSIONS
Effusions as a Tissue Culture Medium
The body fluids constitute an ideal natural tissue culture medium wherein desquamated cells, benign and malignant, may proliferate freely. The temperature, supply of nutrients, oxygen and carbon dioxide are provided by the body of the patient and regulated by much finer homeostatic mechanisms than is possible in vitro. The morphology of cells growing in tissue culture is often different from the cells of origin. This accounts for some of the problems of morphologic cell identification and diagnosis. Specifically, in actively growing tissue cultures, the differentiation of benign from malignant cells may prove to be difficult. Effusions also offer the benefit of study of cells and their interrelationships in a natural setting. Many of the established human cancer cell lines used for in vitro studies are derived from effusions, rather than from solid tissues. Apparently, the ability of these cells to proliferate in the effusion enhances the chances of successful growth in vitro. Thus, the study of effusions constitutes a precious scientific resource that has not been fully explored as yet.
Principal Families of Benign Cells
The principal cell types encountered in the absence of cancer are: mesothelial cells, macrophages (histiocytes), blood cells, and miscellaneous other cells. These are described in the following pages.
Mesothelial Cells
Histology
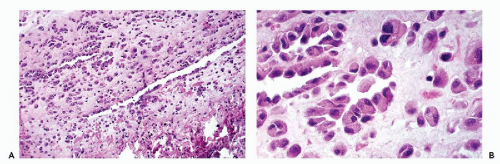
An effusion within the body cavities brings about separation of the two mesothelial layers. As a consequence, the flat mesothelial lining cells become cuboidal in shape. In the presence of an inflammatory process, and sometimes under other circumstances, the orderly arrangement of the mesothelial cells is disturbed. There may be extensive proliferation of mesothelium, both in thickness and in depth, with the formation of several layers of mesothelial cells, sinuses, and channels in continuity with the surface (Fig. 25-6).
Cytology
Aspirates and Scrapes.
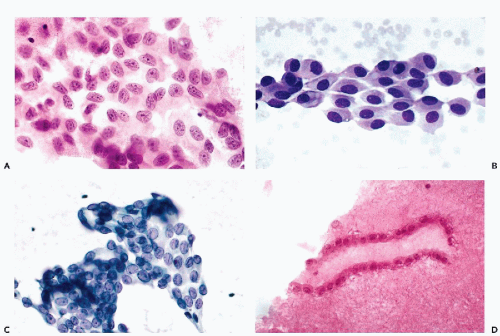
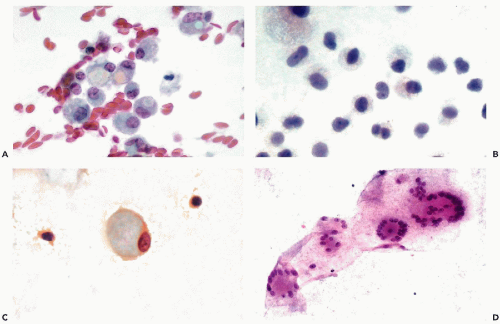
When mesothelial cells are scraped or aspirated by a needle from the surface of the pleural or abdominal cavity, they appear as sheets of polygonal cells, about 20 μm in diameter, that are usually separated from each other by clear gaps or “windows.” The cells have a delicate, yet sharply demarcated, cyanophilic or eosinophilic cytoplasm and round or oval nuclei. The nuclei, generally located in the center of the cell, are of even size, sharply demarcated, slightly granular, and contain one or two, centrally located, readily visible nucleoli (Fig. 25-7A,B). The presence of nucleoli corresponds to the
active metabolic status of these cells that normally produce and maintain the level of fluid lubricating the surfaces of the opposing mesothelial layers. Occasionally, a central fold (crease) may appear in the nucleus (Fig. 25-7C).
active metabolic status of these cells that normally produce and maintain the level of fluid lubricating the surfaces of the opposing mesothelial layers. Occasionally, a central fold (crease) may appear in the nucleus (Fig. 25-7C).
The origin of these cells from the mesothelial surface is best documented in cell blocks that show the linear nature of the clusters cut “on edge” (Fig. 25-7D).
Effusions.
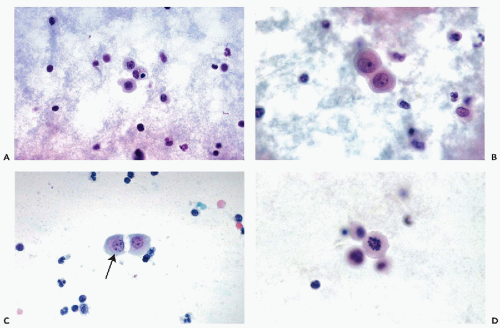
In effusions, the mesothelial cells desquamate from the surface of the lining of the body cavity and accumulate or even proliferate in the body fluids. Free-floating mesothelial cells in fluids appear singly, in doublets, or in clusters of variable sizes and configuration.
Single mesothelial cells in body fluids are usually spherical or oval and measure between 15 and 20 μm in diameter. The cyanophilic or faintly eosinophilic cytoplasm is sharply demarcated (Fig. 25-8A). Under higher magnification, two cytoplasmic zones can be recognized: a perinuclear, denser zone, and a peripheral, clear zone (Fig. 25-8B). The difference is caused by an accumulation of cell organelles in the perinuclear area. A characteristic feature of mesothelial cells in doublets or small linear clusters is the flattening of the opposite cell membranes with formation of a clear gap or “window” (Fig. 25-8B,C). The “windows” are most likely due to microvilli separating the cells. Indeed, Spriggs and Meek (1961) pointed out that free-floating mesothelial cells in effusions may show a narrow brush border that is best observed in air-dried, MGG-stained smears but may also be found in fixed smears and in electron microscopic preparations (see Fig. 25-3). In light microscopy, under high magnification, the mesothelial cells often show a very narrow clear zone surrounding the cell membrane, probably corresponding to the brush border, which is better visualized in air-dried smears. From time to time, mesothelial cells contain eosinophilic cytoplasmic inclusions, identical to those observed in urinary sediment and described in Chapter 22. Cell-in-cell arrangement of mesothelial cells may occur, although this is usually observed in cirrhosis of the liver (see below).
The nuclei of free-floating mesothelial cells are relatively large and occupy about half of the cell diameter. The nucleus is usually centrally located within the cell. The morphologic characteristics of the nucleus are of great diagnostic importance. The nuclear membrane is prominent. The chromatin net is delicate and rather inconspicuous, with a few small, but sharply defined, chromocenters and occasionally one or two tiny nucleoli. Prominent nucleoli, which are a landmark in mesothelial cells in aspirated
samples, are rarely seen in free-floating, desquamated mesothelial cells. In female patients, a single Barr body (sex chromatin body) may be observed (Fig. 25-8B,C).
samples, are rarely seen in free-floating, desquamated mesothelial cells. In female patients, a single Barr body (sex chromatin body) may be observed (Fig. 25-8B,C).
Mesothelial Cell Clusters.
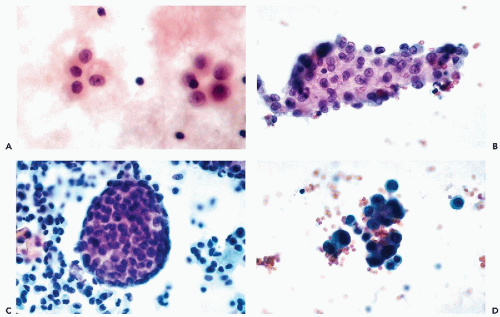
Freely growing mesothelial cells in effusions may also form clusters of various configurations, composed of a variable number of cells (Fig. 25-9). Some of the clusters may be circular, linear, or have a rosette-like configuration (Fig. 25-9A). In single layer clusters, the “windows” separating the mesothelial cells from each other are readily observed. The clusters are readily recognized as benign, so long as the regular configuration of the component cells and their nuclei can be visualized. The outer edges of such clusters are usually composed of rows of cells showing smooth borders or “scalloping.” However, the identification of the clusters as mesothelial in origin becomes much more difficult if the clusters are multilayered, of spherical “papillary” configuration, or poorly fixed with resulting nuclear hyperchromasia (Fig. 25-9B-D). Particularly disturbing are very large spherical clusters that are, from time to time, observed in chronic effusions, particularly in the pericardium (Fig. 25-9C). Spriggs and Jerome (1979) reported such a case and observed the presence of a central core of collagen. Recognizing such clusters as benign is very difficult because malignant tumors, particularly mesotheliomas, may form very similar clusters (see Chap. 26). The dilemma is usually solved by searching for additional clues in single cells that are almost always available in a cytologic preparation. In the absence of clear-cut malignant cells or pertinent clinical history, it is wise to report the papillary clusters with caution.
Diagnostic problems may also occur if the effusion is not processed rapidly. Processing delays may cause the nuclei to stain intensely with hematoxylin (Fig. 25-9D) rendering their interpretation very difficult. In these situations, the similarity of nuclear sizes (and absence of clearly abnormal cells) are in favor of a benign process.
Mitoses in Benign Effusions.
There is no doubt that normal mitotic figures can be observed in benign mesothelial cells growing in effusions (see Fig. 25-8D). Still, mitotic activity in fluids calls for a very careful review of the material to rule out a primary or metastatic malignant tumor. As will be set forth in Chapter 26, in some cases, the inconspicuous tumor cells may resemble mesothelial cells.
The presence of abnormal mitoses has been reported in a very small number of patients in the absence of cancer (Papanicolaou, 1954; Melamed, 1963). Because abnormal mitoses must be considered as a landmark of cancer, it is inevitable that in such extremely rare cases, an erroneous diagnosis will be rendered.
Special Stains in Identification of Mesothelial Cells
Conventional Stains
The differentiation of mesothelial cells from morphologically similar cancer cells has been the subject of inquiry for many years. In 1959, Nathan Chandler Foot examined the specificity of the periodic acid-Schiff reagent (PAS) for the mesothelial cell. He found that the cytoplasm of most mesothelial cells contained PAS-positive granules concentrated at the periphery and representing, in all likelihood, neutral mucopolysaccharides. Although the cytoplasm of some cancer cells stained with PAS, the red color was diffuse and not confined to the granules. Mavrommatis (1964) generally confirmed the observations of Foot. However, Pfitzer (1966) denied any diagnostic value in the use of PAS technique. In my experience, the PAS method is rarely of diagnostic assistance in a critical morphologic situation.
Of greater use are stains identifying mucin, such as mucicarmine. Mucicarmine-positive staining has never been observed in this laboratory in mesothelial cells whereas it is an excellent indicator of mucin-producing carcinomas.
Antibodies
Many attempts have been made to identify cancer cells in effusions with monoclonal antibodies. A monoclonal antibody recognizing mesothelial cells, developed by Singh, was used by us in the identification of a mesothelioma of the tunica vaginalis of the testis (Japko et al, 1982) but there is no evidence that this antibody became commercially available. Davidson et al (2001) observed that antibodies to desmin mark the mesothelial cells more intensely than cancer cells. Panels of antibodies used in the differential diagnosis of mesotheliomas from benign mesothelial cells or other tumors are discussed in Chapter 26 and Chapter 45.
Macrophages (Histiocytes)
The macrophages are of bone marrow origin and represent transformed monocytes. The proof of this origin is discussed in Chapter 19. In the past, it had been thought that the presence of macrophages in effusions reflected a chronic inflammatory process. Current evidence strongly suggests that macrophages are ubiquitous cells that can also be observed in the presence of cancer and under other circumstances as well. Using immunocytochemistry and flow cytometry with the marker CD14 to identify monocyte/macrophage cell populations in benign and malignant effusions, Risberg et al (2001) confirmed that these cells are present in virtually all effusions and often comprise a surprisingly high proportion of all cells, up to 85%. The presence of macrophages was somewhat higher in female than male patients but was unrelated to the presence or absence of cancer. The relationship of macrophages to cancer cells is discussed in Chapter 26.
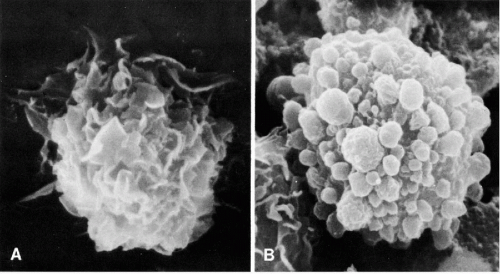
Studies based on scanning and transmission electron microscopy of fluids disclosed that many cells that have been hitherto classified as of mesothelial origin, particularly cells with large cytoplasmic vacuoles, belong to the family of macrophages (Domagala and Woyke, 1975; Murad, 1973; Domagala and Koss, 1977). The surface configuration of the two cell types in scanning electron microscopy discloses major differences. The mesothelial cells are characterized by the presence of regular short microvilli or a mixture of microvilli with bleb-like surface structures, the latter characterizing older cells. The macrophages have a very characteristic surface configuration, wherein the cell membrane forms folds or ridges (Fig. 25-10). Although mesothelial cells contain some lysosomes (Cotran and Karnovsky, 1968), they do not possess the elaborate lysosomal apparatus characterizing the macrophages.
In effusions, the number of macrophages is extremely variable, depending on the condition of the patient. In some infectious effusions, notably in Legionella micdadei infection, the macrophages may be the dominant cell population.
The macrophages are usually seen as mono- or binucleated cells similar in size to mesothelial cells (15 to 20 μm in diameter). These cells usually occur singly or in loosely arranged groups and do not show cytoplasmic molding or “windows.” The macrophages are characterized by foamy cytoplasm, studded with minute vacuoles, or containing phagocytized particles, and a cell border that readily blends with the smear background, in contrast with the sharply demarcated mesothelial cells (see Fig. 25-8A; Fig. 25-11A). The cytoplasm of the macrophages may become markedly distended with large, clear vacuoles (Fig. 25-11B).
The position of the nuclei of mono- or binucleated macrophages is variable. In most macrophages, the nuclei are located in the peripheral portion of the cell and sometimes stain somewhat denser than the nuclei of mesothelial cells in the same field. Kidney-shaped, indented nuclei with finely granular chromatin, and sometimes tiny nucleoli, are common in macrophages and uncommon in mesothelial cells. In vacuolated macrophages, the nuclei are often compressed and pushed to the periphery (Fig. 25-11C). However, the nuclear configuration or staining properties are of limited value in separating macrophages from mesothelial cells.
Large multinucleated macrophages, either foreign body giant cells or Langhans’ type cells, may occasionally be observed. Such cells are usually observed in fluids as a reaction to foreign material after a surgical intervention, in granulomatous inflammations, or after radiotherapy, presumably as a reaction to necrosis of cells (Fig. 25-11D). Ordoñez et al (1998) reported that the so-called nodular mesothelial hyperplasia is composed of macrophages (see Chap. 26). Clusters of macrophages were described by Choi and Song (2001) in pleural fluid in a case of this rare disorder.
Phagocytic activity and lysosomal activity are characteristic functions of macrophages. These features may be used to good advantage in the identification of these cells in light microscopy (see Fig. 25-8A; Fig. 25-11A). Pigments, organic or inorganic particle or cell engulfment by macrophages, may be observed (see below).
Stains for enzymes, such as acid phosphatase or esterases, may document the intense lysosomal activity in macrophages. None of these functions is normally observed in mesothelial cells but there are rare exceptions, when mesothelial cells acquire phagocytic properties. In such cases, the distinction between macrophages and mesothelial cells is impossible in light microscopy. In unfixed smears from fresh effusion, the macrophages may be identified by supravital staining with neutral red or Janus green which is absorbed by macrophages (and some leukocytes) but not by mesothelial cells (Foot and Holmquist, 1958). Identification of macrophages in effusions with the specific cell surface marker CD14 was reported by Risberg et al (2001).
Blood Cells
Erythrocytes
The presence of intact red blood cells in body fluids is usually caused by a traumatic tap. In hemorrhagic effusions, fresh and degenerated erythrocytes are usually seen against a background of fibrin. Sickle cell anemia may be identified in fluids in the form of sickle-shaped erythrocytes (Dekker et al, 1975).
Erythrophagocytosis
Ingestion of the patient’s own erythrocytes by macrophages in pleural or ascitic fluids may be occasionally observed, for example, in dialysis ascites (see below). Several other examples have been observed by us under a variety of circumstances (Fig. 25-12A; see Fig. 25-22A). It is not known whether the erythrocyte surface has to be modified for these cells to be phagocytosed. Erythrophagocytosis has been observed in a rare disorder defect of lysosomes occurring in macrophages, known as the Chediak-Higashi syndrome (Valenzuela et al, 1976) and may be observed in some forms of malignant lymphoma (see Chap. 26). Zaharopoulos (2001) observed erythrophagocytosis in pleural fluid from a patient with autoimmune hemolytic anemia, induced by Epstein-Barr virus.
Leukocytes
Lymphocytes.
Leukocytes in effusions are extremely common. In chronic effusions of long-standing, lymphocytes may be the dominant population of leukocytes (Fig. 25-12B). If the lymphocytes are numerous and, especially if no other leukocytes are present, the possibility of a chylous effusion caused by a rupture of the thoracic duct, tuberculosis, chronic lymphocytic leukemia, or well-differentiated malignant lymphoma should be investigated. Typing and enumeration of B and T lymphocytes and their subtypes may be of diagnostic value. The implications of this observation in reference to cancer in effusions is discussed in Chapter 26.
Granulocytes.
Polymorphonuclear neutrophilic leukocytes (polys) invariably indicate an inflammatory process, which may be secondary to infection, cancer or other disorders. Eosinophilic leukocytes may be seen in eosinophilic pleural effusions (see below) and in a variety of inflammatory processes. They are not uncommon in tuberculosis and are rarely seen in Hodgkin’s disease.
Plasma Cells.
Plasma cells may be noted in chronic inflammatory processes in multiple myeloma and in Hodgkin’s disease (see Chap. 26).
Megakaryocytes
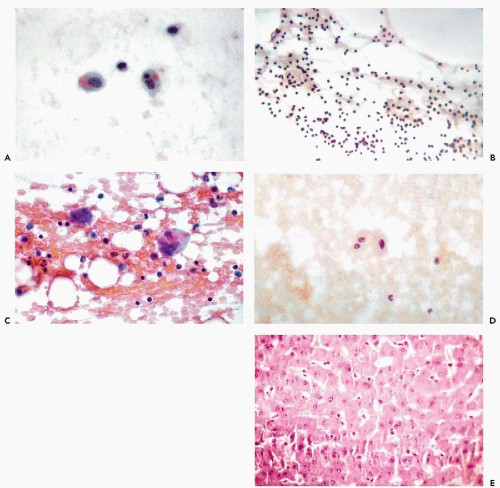
Calle (1968) was the first to observe these cells in the abdominal fluid of a female patient with myeloid metaplasia. Extramedullary hematopoesis appears to be the common denominator of cases reported by others (Vilaseca et al, 1981; Pedio et al, 1985; Silverman, 1985). Barziotas and Naylor (1986) observed megakaryocytes in a bloody pleural effusion in a patient with overdose of an anticoagulant (Fig. 25-12C).
Abnormal megakaryocytes may signal a serious hematopoietic disorder (see Chap. 26).
Other Hematopoietic Cells
Busmanis et al (1998) reported the presence of a broad spectrum of hematopoietic cells, mimicking leukemia, in the pleural fluid in a patient receiving granulocyte-stimulating factor after chemotherapy.
Other Benign Cells Encountered in Effusions
Anitschkow’s Cells
Cells similar to Anitschkow’s myocytes, that have a very characteristic central bar of chromatin, from which radiate numerous short lateral processes (caterpillar nuclei), have been noted in a rare case of effusion of long standing and of unknown etiology (see Fig. 25-7C). Molina and Schnadig (2001) observed such cells in six of fourteen pericardial scrapings and concluded that these are modified mesothelial cells. Similar cells may be observed in oral and conjunctival squamous cells (see Chaps. 21 and 41).
Liver Cells
Liver cells, singly or in sheets, may be observed if this organ is penetrated accidentally while fluids are aspirated from the right pleural cavity (Fig. 25-12D,E). The identification is quite easy because of the large size of the cells and their abundant, faintly vacuolated cytoplasm surrounding large single or double nuclei. The identity of the cells may be confirmed in cell blocks. Tiny, green-staining intracytoplasmic bile deposits may be observed in fresh preparations. In older preparations, the bile forms green or yellow amorphous masses.
Cells Derived From the Respiratory Tract
Readily recognizable ciliated bronchial cells and dust-containing macrophages in pleural fluid are the result of injury to lung tissue during tapping, and may suggest a bronchopleural fistula or, exceptionally, a mediastinal teratoma with elements of the respiratory tract.
Squamous Cells
Benign squamous cells derived from the epidermis of the skin are very rarely seen in effusions. In general, the presence of squamous cells in effusions, regardless of their morphology, suggests a squamous carcinoma (see Chap. 26). In a case described by Cobb et al (1985), benign squamous cells, anucleated squames, and hair shafts were observed in pleural fluid from a boy with ruptured benign cystic teratoma (dermoid cyst) of the anterior mediastinum.
Fat Cells and Striated Muscle
These cells, originating in subcutaneous fat and muscle, may be noted occasionally. They are incidental to tapping.
Pigments
Hemosiderin
Cytoplasmic hemosiderin deposits in the form of fine granular golden-brown pigment are found in macrophages in effusions in chronic hemorrhagic pleurisy or pericardial effusion. The large hemosiderin deposits may obscure the nuclei of macrophages and may mimic melanin accumulation and lead to a mistaken diagnosis of a malignant melanoma. Hemosiderin stains green with iron stains which can be used for identification of this pigment (see Chap. 19).
Melanin
Melanin may be observed in macrophages as a diffuse or finely granular brown cytoplasmic stain in the presence
of metastatic malignant melanoma. Allen et al (1997) observed melanin accumulation in macrophages in a pleural effusion in a patient with melanosis coli, a benign condition in which melanin accumulates in the epithelial cells of the colon. Melanin can be identified with a number of monoclonal antibodies such as HMB45 and MART-1 (Beaty et al, 1997) (see also Chap. 26).
of metastatic malignant melanoma. Allen et al (1997) observed melanin accumulation in macrophages in a pleural effusion in a patient with melanosis coli, a benign condition in which melanin accumulates in the epithelial cells of the colon. Melanin can be identified with a number of monoclonal antibodies such as HMB45 and MART-1 (Beaty et al, 1997) (see also Chap. 26).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree